Free Radical Scavenging Activity of Boron and Vitamin C in Nitrite-Induced Hemoglobin Oxidation Model: In vitro and in vivo Studies
DOI:
https://doi.org/10.54133/ajms.v5i.202Keywords:
Boron, Methemoglobinemia, Nitrite, Oxidative stress, Vitamin CAbstract
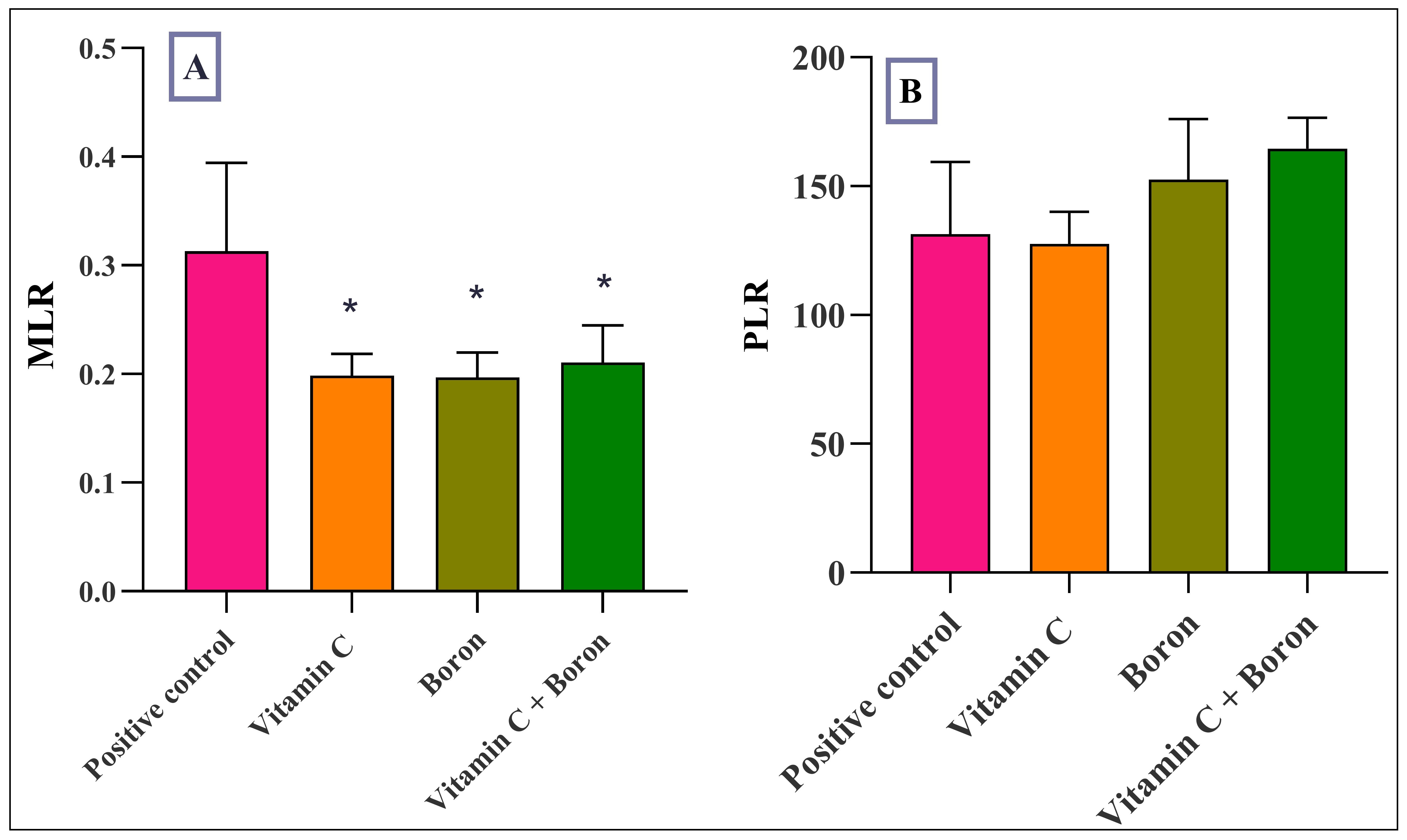
Objective: To investigate the dose-response relationship of the free radical-scavenging activities of boron and vitamin C in nitrite-induced hemoglobin oxidation in vitro and in vivo. Method: Different concentrations of boron and vitamin C were added to a hemolysate challenged with nitrite to induce methemoglobinemia (MetHb), and the most effective dose of boron and vitamin C was used before and after different intervals of inducing Hb oxidation, and the production of MetHb was monitored using a spectrophotometer. The effective doses of boron and vitamin C, alone and in combination, were administered to rats before challenging them with an oral dose of 100 mg/kg sodium nitrite. Results: In vitro results indicated that different concentrations of boron and vitamin C attenuated MetHb formation, with the maximum effect achieved at 0.08mg/L and 10mg/L, respectively. Moreover, when these doses were used at different time intervals, a maximum effect was achieved when added 10 min before nitrite. The in vivo results demonstrated a significant reduction in methemoglobin formation in rats treated with boron and vitamin C alone. The hematological markers were not changed except for the platelet levels, which increased in the boron-treated and combination groups. The monocyte-to-lymphocyte ratio decreased significantly in all treatment groups compared with the positive control group. Conclusion: Boron protects against Hb oxidation induced by nitrite, and a potentiated effect has been achieved with the combination of vitamin C.
Downloads
References
Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive oxygen species generation and stherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(5):e41-e52. doi:10.1161/ATVBAHA.117.309228. DOI: https://doi.org/10.1161/ATVBAHA.117.309228
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20(9):689-709. doi: 10.1038/S41573-021-00233-1. DOI: https://doi.org/10.1038/s41573-021-00233-1
Antonucci S, Di Lisa F, Kaludercic N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium. 2021;94. doi:10.1016/J.CECA.2020.102344 DOI: https://doi.org/10.1016/j.ceca.2020.102344
Li Y, Yang J, Sun X. Reactive oxygen species-based nanomaterials for cancer therapy. Front Chem. 2021;9. doi:10.3389/FCHEM.2021.650587 DOI: https://doi.org/10.3389/fchem.2021.650587
Aziz TA. Concentration-dependent antioxidant activity of pentoxifylline in nitrite-induced hemoglobin oxidation model. Iraqi J Pharm Sci. 2011;20(1):66-69. doi: 10.31351/vol20iss1pp66-69. DOI: https://doi.org/10.31351/vol20iss1pp66-69
Marouf BH, Zalzala MH, Al-Khalifa II, Aziz TA, Hussain SA. Free radical scavenging activity of silibinin in nitrite-induced hemoglobin oxidation and membrane fragility models. Saudi Pharm J. 2011;19(3):177. doi:10.1016/J.JSPS.2011.03.006. DOI: https://doi.org/10.1016/j.jsps.2011.03.006
Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015;5(4):216. doi:10.5662/WJM.V5.I4.216 DOI: https://doi.org/10.5662/wjm.v5.i4.216
Tijjani H, Omar AA, Mohammed A, Habibu FM, Maina YB, Musa A. In vitro antioxidant activities and blood protective effects of aqueous extracts of Xylopia aethiopica L. whole seed and pod. Niger J Biochem Mol Biol. 2022;37(1):48-57. doi:10.2659/NJBMB.2022.8.
Wang W, Kang PM. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants (Basel, Switzerland). 2020;9(12):1-25. doi:10.3390/ANTIOX9121292. DOI: https://doi.org/10.3390/antiox9121292
Njälsson R, Norgren S. Physiological and pathological aspects of GSH metabolism. Acta Paediatr. 2005;94(2):132-137. doi:10.1111/J.1651-2227.2005.TB01878.X. DOI: https://doi.org/10.1080/08035250410025285
Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5(1):51. doi:10.1038/NPROT.2009.197. DOI: https://doi.org/10.1038/nprot.2009.197
Dean DE, Looman KB, Topmiller RG. Fatal methemoglobinemia in three suicidal sodium nitrite poisonings. J Forensic Sci. 2021;66(4):1570-1576. doi: 10.1111/1556-4029.14689. DOI: https://doi.org/10.1111/1556-4029.14689
Jiheel MJ, Arrak JK. Role of DPP (Phoenix dactylifera L.) extract on ameliorating the incidence of hemoglobin oxidation induced by sodium nitrite. Kufa J Vet Med Sci. 2015;6(2):161-169. doi: 10.36326/kjvs/2015/v6i23995. DOI: https://doi.org/10.36326/kjvs/2015/v6i23995
Kang C, Kim DH, Kim T, Lee SH, Jeong JH, Lee SB, et al. Therapeutic effect of ascorbic acid on dapsone-induced methemoglobinemia in rats. Clin Exp Emerg Med. 2018;5(3):192-198. doi: 10.15441/ceem.17.253. DOI: https://doi.org/10.15441/ceem.17.253
Lien YH, Lin YC, Chen RJ. A case report of acquired methemoglobinemia rescued by veno-venous extracorporeal membrane oxygenation. Medicine (Baltimore). 2021;100(15):e25522. doi: 10.1097/MD.0000000000025522. DOI: https://doi.org/10.1097/MD.0000000000025522
Albuquerque RV, Malcher NS, Amado LL, Coleman MD, Dos Santos DC, Borges RS, et al. In vitro protective effect and antioxidant mechanism of resveratrol induced by dapsone hydroxylamine in human cells. PLoS One. 2015;10(8):e0134768. doi: 10.1371/journal.pone.0134768. DOI: https://doi.org/10.1371/journal.pone.0134768
Krukoski DW, Comar SR, Claro LM, Leonart MS, do Nascimento AJ. Effect of vitamin C, deferoxamine, quercetin and rutin against tert-butyl hydroperoxide oxidative damage in human erythrocytes. Hematology. 2009;14(3):168-172. doi: 10.1179/102453309X402296. DOI: https://doi.org/10.1179/102453309X402296
Asgary S, Naderi GH, Askari N. Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp Clin Cardiol. 2005;10(2):88.
Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. 2012;64(1-2):93-101. doi: 10.1016/J.ETP.2010.06.011. DOI: https://doi.org/10.1016/j.etp.2010.06.011
Kucukkurt I, Ince S, Demirel HH, Turkmen R, Akbel E, Celik Y. The effects of boron on arsenic-induced lipid peroxidation and antioxidant status in male and female rats. J Biochem Mol Toxicol. 2015;29(12):564-571. doi:10.1002/JBT.21729. DOI: https://doi.org/10.1002/jbt.21729
Tutunchi H, Mobasseri M, Pourmoradian S, Soleimanzadeh H, Kafil B, Akbari N, et al. Assessment of boron-containing compounds and oleoylethanolamide supplementation on the recovery trend in patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):890. doi: 10.1186/s13063-020-04820-2. DOI: https://doi.org/10.1186/s13063-020-04820-2
Özkan E, Karabağ Çoban F. Investigation of boron effect on trace elements and antioxidant capacity in paracetamol-induced nephrotoxicity model. J Turkish Vet Med Soc. 2020;91(1):25-35. doi:10.33188/VETHEDER.557918. DOI: https://doi.org/10.33188/vetheder.557918
Ameen H, Hussain S, Ahmed Z, Aziz T. Anti-inflammatory effects of boron alone or as adjuvant with dexamethasone in animal models of chronic and granulomatous inflammation. Int J Basic Clin Pharmacol. 2015;4(4):701-707. doi:10.18203/2319-2003.ijbcp20150376. DOI: https://doi.org/10.18203/2319-2003.ijbcp20150376
I. Scorei R, Popa R. Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anticancer Agents Med Chem. 2010;10(4):346-351. doi:10.2174/187152010791162289. DOI: https://doi.org/10.2174/187152010791162289
Nzietchueng RM, Dousset B, Franck P, Benderdour M, Nabet P, Hess K. Mechanisms implicated in the effects of boron on wound healing. J Trace Elem Med Biol. 2002;16(4):239-244. doi:10.1016/S0946-672X(02)80051-7. DOI: https://doi.org/10.1016/S0946-672X(02)80051-7
Kurtoglu V, Kurtoglu F, Akalin PP. The effects of various levels of boron supplementation on live weight, plasma lipid peroxidation, several biochemical and tissue antioxidant parameters of male mice**: Effects of boron on performance, antioxidant and some metabolites of mice. J Trace Elem Med Biol. 2018;49:146-150. doi: 10.1016/j.jtemb.2018.05.013. DOI: https://doi.org/10.1016/j.jtemb.2018.05.013
Hathazi D, Scurtu F, Bischin C, Mot A, Attia AAA, Kongsted J, et al. The reaction of oxy hemoglobin with nitrite: Mechanism, antioxidant-modulated effect, and implications for blood substitute evaluation. Molecules. 2018;23(2):350. doi: 10.3390/molecules23020350. DOI: https://doi.org/10.3390/molecules23020350
Hussain AA, Vinoth K, Mani VM. Protective effect of vitamin C on deltamerhrin induced oxidative stress in human erythrocytes- An in vitro study. Int J Sci Human. 2018;4(1):26-39.
GluhchevaY, Ivanov I, Petrova E, Pavlova E, Vladov I. Sodium nitrite-induced hematological and hemorheological changes in rats. Series on Biomechanics. 2012; 27 (3-4): 53-58.
Kadhum HJ, Al-Diwan MA, Al-Jadaan SA. Bis4-(4-́ hydroxy-3-́methoxy benzylidine aminophenyl) telluride prevents sodium nitrite induced changes in hematological parameters of adult's male rats. Merit Res J Med Med Sci. 2022;10(1):009-014. doi: 10.5281/zenodo.5910650.
Fouad SS, Mohi-Eldin MM, Haridy MA, Khalil AM. Ameliorative effects of ascorbic acid (Vit. C) against sodium nitrite toxicity in albino rats: Hematological, biochemical and histopathological studies. Am-Euras J Toxicol Sci. 2017;9(1):01-06. doi: 10.5829/idosi.aejts.2017.01.06.
Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: A mechanistic study. J Biol Chem. 2008;283(15):9615-9622. doi: 10.1074/jbc.M705630200. DOI: https://doi.org/10.1074/jbc.M705630200
Umbreit J. Methemoglobin—It’s not just blue: A concise review. Am J Hematol. 2007;82:134-144. doi: 10.1002/ajh.20738. DOI: https://doi.org/10.1002/ajh.20738
Kumar MS, Unnikrishnan MK, Patra S, Murthy K, Srinivasan kk. Naringin and naringenin inhibit nitrite-induced methemo- globin formation. Pharmazie. 2003;58(8):564-566.
Ince S, Kucukkurt I, Demirel HH, Acaroz DA, Akbel E, Cigerci IH. Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere. 2014;108:197-204. doi: 10.1016/j.chemosphere.2014.01.038. DOI: https://doi.org/10.1016/j.chemosphere.2014.01.038
Basol N, Ozmen C, Ocakli S, Cetin S. Evaluation of the effects of curcumin, erdosteine, vitamin E and vitamin C on paracetamol toxicity. Med Sci. 2022;11(2):465-470. doi: 10.5455/medscience.2021.08.269. DOI: https://doi.org/10.5455/medscience.2021.08.269
Al-Gareeb AA., Mohammed GF. Hepatoprotective effects of vitamin c against methotrexate induced acute liver injury: an experimental study. Bull Pharm Sci Assiut Univ. 2022;45(1):459-468. DOI: https://doi.org/10.21608/bfsa.2022.239624
Dotsch J, Demirakca S, Cryer A, Hanze J, Kuhl PG, Rascher W. Reduction of NO-induced methemoglobinemia requires extremely high doses of ascorbic acid in vitro. Intens Care Med. 1998;24:612-615. doi:10.1007/s001340050623. DOI: https://doi.org/10.1007/s001340050623
Calabrese EJ, Moore GS, McCarthy MS. The effect of ascorbic acid on nitrite-induced methemoglobin formation in rats, sheep, and normal human erythrocytes. Regul Toxicol Pharmacol. 1983;3(3):184-188. doi:10.1016/0273-2300(83)90026-0. DOI: https://doi.org/10.1016/0273-2300(83)90026-0
Hassan SMH, Zaglool NF, El-Shamy SA. Comparative studies on turmeric and vitamin C on sodium nitrite treated rats. AJVS. 2018;56(1):56-68. doi: 10.5455/ajvs.278511. DOI: https://doi.org/10.5455/ajvs.278511
Amin RA, Elsabagh RA, Amin A. Protective effects of ascorbic acid and garlic oil against toxic effects induced by sodium nitrite as meat additive in male rats. Global Veterinaria. 2016;16(6):508-524. doi: 10.5829/idosi.gv.2016.16.06.10393.
Bolt HM, Duydu Y, Basaran N, Golka K. Boron and its compounds: current biological research activities. Arch Toxicol. 2017; 91:2719-2722. doi: 10.1007/s00204-017-2010-1. DOI: https://doi.org/10.1007/s00204-017-2010-1
Ayhanci A, Lafçi n, Musmul A, Gür F, Sezer CV, Şahi̇n IK , et al. The protective effects of selenium and boron against cyclophosphamide-induced bone marrow and blood toxicity: An in vivo study. Biol Divers Conserv. 2022;15(2):256-264. doi: 10.46309/biodicon.2022.1124346. DOI: https://doi.org/10.46309/biodicon.2022.1124346
Pizzorno L. Nothing boring about boron. Integr Med (Encinitas). 2015;14(4):35-48.
Donoiu I, Militaru C, Obleagă O, Hunter JM, Neamţu J, Biţăe A, et al. Effects of boron-containing compounds on cardiovascular disease risk factors – A review. J Trace Elem Med Biol. 2018;50:47-56. doi: 10.1016/j.jtemb.2018.06.003. DOI: https://doi.org/10.1016/j.jtemb.2018.06.003
Cakir S, Eren M, Senturk M, Sarica ZS. The Effect of boron on some biochemical parameters in experimental diabetic rats. J Biol Trace Elem Res. 2017. doi: 10.1007/s12011-017-1182-0. DOI: https://doi.org/10.1007/s12011-017-1182-0
Bhasker TV, Gowda NKS, Mondal S, Krishnamoorthy P, Pal DT, Mor A, et al. Boron influences immune and antioxidant responses by modulating hepatic superoxide dismutase activity under calcium deficit abiotic stress in Wistar rats. J Trace Elem Med Biol. 2016;36:73-79. doi: 10.1016/j.jtemb.2016.04.007. DOI: https://doi.org/10.1016/j.jtemb.2016.04.007
Coban F K, Ince S, Kucukkurt I, Demirel HH, and Hazman O. Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem Toxicol. 2014;1-9. doi: 10.3109/01480545.2014.974109. DOI: https://doi.org/10.3109/01480545.2014.974109
Ayhanci A, Tanriverdi DT, Sahinturk V, Cengiz M, Appak-Baskoy S, Sahin IK. Protective effects of boron on cyclophosphamide-induced bladder damage and oxidative stress in rats. Biol Trace Elem Res. 2019. doi: 10.1007/s12011-019-01969-z. DOI: https://doi.org/10.1007/s12011-019-01969-z
Hu Q, Li S, Qiao E, Tang Z, Jin E, Jin G, et al. Effects of boron on structure and antioxidative activities of spleen in rats. Biol Trace Elem Res. 2014. doi 10.1007/s12011-014-9899-5. DOI: https://doi.org/10.1007/s12011-014-9899-5
Keklik E, Keklik M, Bakkaloglu U, Yuruk M, Coksevim B. The Effect of Borax on Hematological Parameters and Immunoglobulin Values in Rats. West Indian Med J. 2016;1-14. doi:10.7727/wimj.2016.359. DOI: https://doi.org/10.7727/wimj.2016.359
Durmus I, Ince S, Salim MN, Eryavuz A, Kucukkurt I. Effects of boron administration on hematological parameters in rats given gentamicin. Kocatepe Vet J. 2018;11(2):140-147. DOI: https://doi.org/10.30607/kvj.394370
Iztleuov M, Zhexenova A, Abdoldayeva S, Iztleuova G, Zhoengaloeva A, Altayeva A, et al. Influence of boron compounds on chromium-induced hemorheology disorders in rats. Biomed Pharmacol J. 2017;10(4):1779-1786. doi: 10.13005/bpj/1292. DOI: https://doi.org/10.13005/bpj/1292
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











