Cytotoxicity of L-Methioninase Purified from Clinical Isolates of Pseudomonas Species in Cancer Cell Lines
DOI:
https://doi.org/10.54133/ajms.v6i1.405Keywords:
Anticancer activity, Cytotoxicity, L-methioninase, Pseudomonas Spp.Abstract
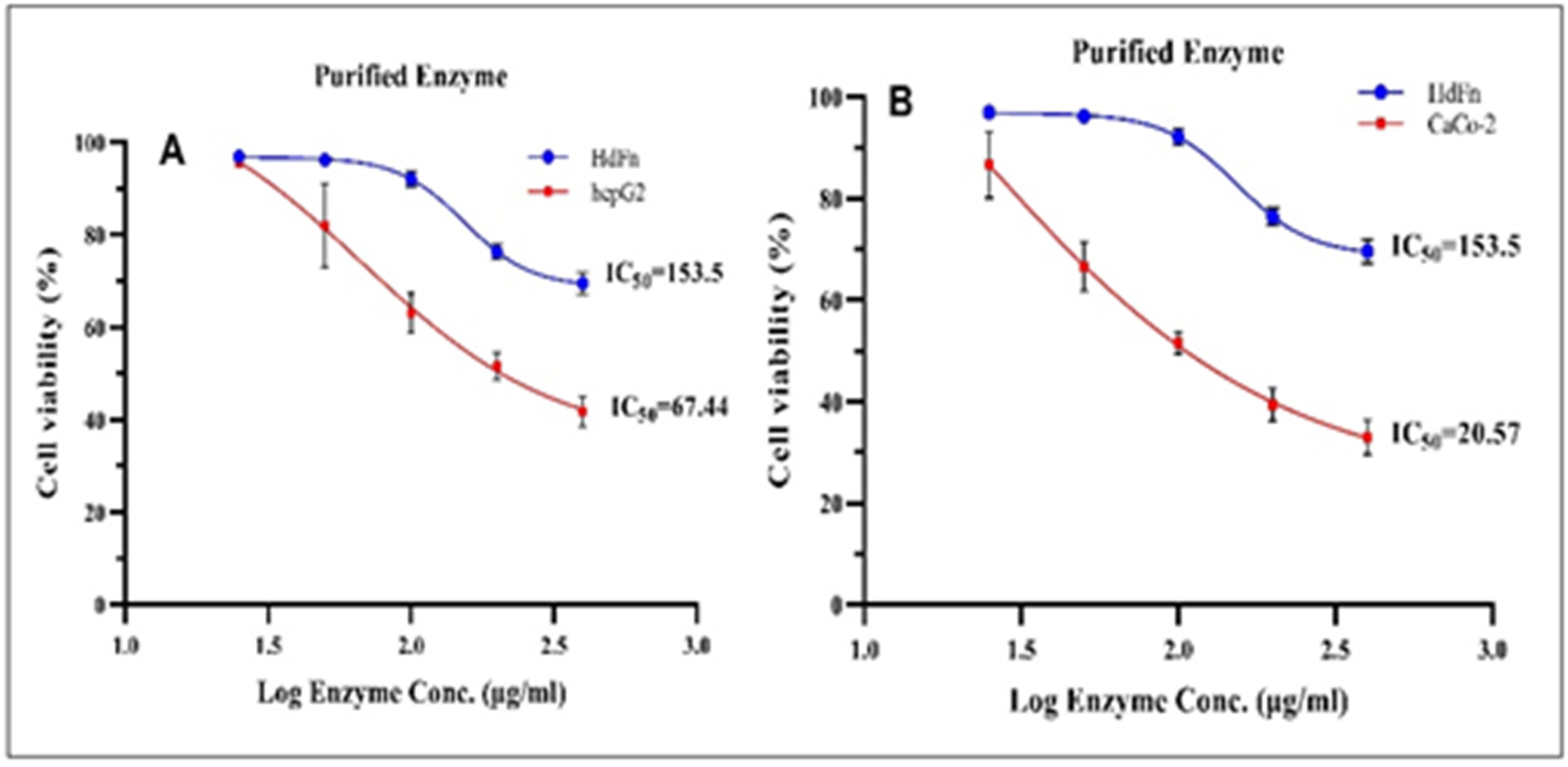
Background: L-methioninase is an enzyme that was found in Pseudomonas spp. It changes L-methionine into α-ketobutyrate, ammonia, and methanethiol. It has been thoroughly investigated for possible antibacterial and anticancer activities. Objective: The goal is to find out how well L-methioninase kills colon CaCo-2 and liver HepG2 cancer cells. Methods: The enzyme was taken from 33 different types of Pseudomonas, and their ability to make L-methioninase was tested on M9 media that had been changed. An MTT assay was used to evaluate the cytotoxic activity of HepG2 and CaCo2 cell lines. Results: Only 15 isolates were able to make L-methioninase. The best isolate had a specific activity of 1.4 μg/U protein. The enzyme's cytotoxicity showed that it stopped the growth of the HepG-2 cell line with an IC50 of 67.44 μg/ml, compared to an IC50 of 140.0 μg/ml for the crude enzyme, and it stopped the growth of the CaCo-2 cell line with an IC50 of 20.57 μg/ml, compared to 154.3 μg/ml for the crude enzyme. Conclusions: Isolation of L-methioninase from microbial sources can be an efficient source to produce this cytotoxic agent.
Downloads
References
Shalev O, Ashkenazy H, Neumann M, Weigel D. Commensal Pseudomonas protect Arabidopsis thaliana from a coexisting pathogen via multiple lineage-dependent mechanisms. Multidisciplin J Microbiol Ecol. 2022;16(5):1235-1244. doi: 10.1038/s41396-021-01168-6.
Rossi E, La Rosa R, Bartell JA, Marvig RL, Haagensen JA, Sommer LM, et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat Rev Microbiol. 2021;19(5):331-342. doi: 10.1038/s41579-020-00477-5.
Maggi, Maristella, and Claudia Scotti. "Enzymes in metabolic anticancer therapy." Therapeutic Enzymes: Function and Clinical Implications .2019: 173-199. doi: 10.1007/978-981-13-7709-9_9.
Abdelraof, Mohamed, et al. "Statistically optimized production of extracellular l-methionine γ-lyase by Streptomyces Sp. DMMMH60 and evaluation of purified enzyme in sub-culturing cell lines. Biocatalysis Agricult Biotechnol. 2019;18(1):101074. doi: 10.1016/j.bcab.2019.101074.
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trend Biochem Sci. 2010;35(8):427-433. doi: 10.1016/j.tibs.2010.05.003.
Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A, Maurizis JC, et al. Methionine dependency and cancer treatment. Cancer Treat Rev. 2003;29(6):489-499. doi: 10.1016/S0305-7372(03)00118-X.
Sun X, Yang Z, Li S, Tan Y, Zhang N, Wang X, et al. In vivo efficacy of recombinant methioninase is enhanced by the combination of polyethylene glycol conjugation and pyridoxal 5′-phosphate supplementation. Cancer Res. 2003;63(23):8377-8383. PMID: 14678999.
Selim MH, Elshikh HH, Saad MM, Mostafa EE, Mahmoud MA. Purification and characterization of a novel thermo stable L-methioninase from Streptomyces sp. DMMMH4 and its evaluation for anticancer activity. J Appl Pharm Sci. 2016;6(7):053-060. doi: 10.7324/JAPS.2016.60708.
Salim N, Santhiagu A, Joji K. Process modeling and optimization of high yielding L-methioninase from a newly isolated Trichoderma harzianum using response surface methodology and artificial neural network coupled genetic algorithm. Biocatal Agricult Biotechnol. 2019;17:299-308. doi: 10.1016/j.bcab.2018.11.032.
Sundar WA, Nellaiah H. Production of methioninase from Serratia marcescens isolated from soil and its anti-cancer activity against Dalton’s Lymphoma Ascitic (DLA) and Ehrlich Ascitic Carcinoma (EAC) in Swiss albino mice. Trop J Pharm Res. 2013;12(5):699-704. doi: 10.4314/tjpr.v12i5.6.
Freshney RI. Culture of animal cells: a manual of basic technique and specialized applications. John Wiley & Sons; 2015.
Al-Saily HM, Al-Halbosiy M, Al-Hady FN. Cytotoxic and apoptotic effects of cyproterone acetate against cancer cells and normal cells. J Biotechnol Res Center. 2019;13(1):68-74.
Selim, MH, Karm Eldin, EZ, Saad MM, Mostafa ESE, Shetia YH, Anise AAH. Purification, characterization of L-methioninase from Candida tropicalis, and its application as an anticancer. Biotechnol Res Int. 2015. doi: 10.1155/2015/173140.
Sundar WA, Nellaiah H. A rapid method for screening of methioninase producing Serratia marcescens species from soil. Int J Pharmacy Pharm Sci. 2013;5(2):426-427.
El-Sayed AS, Shouman SA, Nassrat HM. Pharmacokinetics, immunogenicity and anticancer efficiency of Aspergillus flavipes L-methioninase. Enzy Microb Technol. 2012;51(4):200-210. doi: 10.1016/j.enzmictec.2012.06.004.
Suganya K, Govindan K, Prabha P, Murugan M. An extensive review on L-methioninase and its potential applications. Biocatal Agricult Biotechnol. 2017;12:104-115. doi: 10.1016/j.bcab.2017.09.009.
Salim N, Santhiagu A, Joji K. Purification, characterization and anticancer evaluation of l-methioninase from Trichoderma harzianum. 3 Biotech. 2020;10(11):501. doi: 10.1007/s13205-020-02494-w.
Hendy MH, Hashem AH, Sulieman WB, Sultan MH, Abdelraof M. Purification, Characterization and anticancer activity of L-methionine γ-lyase from thermo-tolerant Aspergillus fumigatus. Microb Cell Fact. 2023;22(1):1-1. doi:10.1186/s12934-023-02019-z.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











