Expression of BAX and eNOS in Rabbit Pancreatic Tissues Injured by Hydrocortisone
DOI:
https://doi.org/10.54133/ajms.v6i1.566Keywords:
BAX, eNOS, Hydrocortisone, Immunohistochemistry, PancreasAbstract
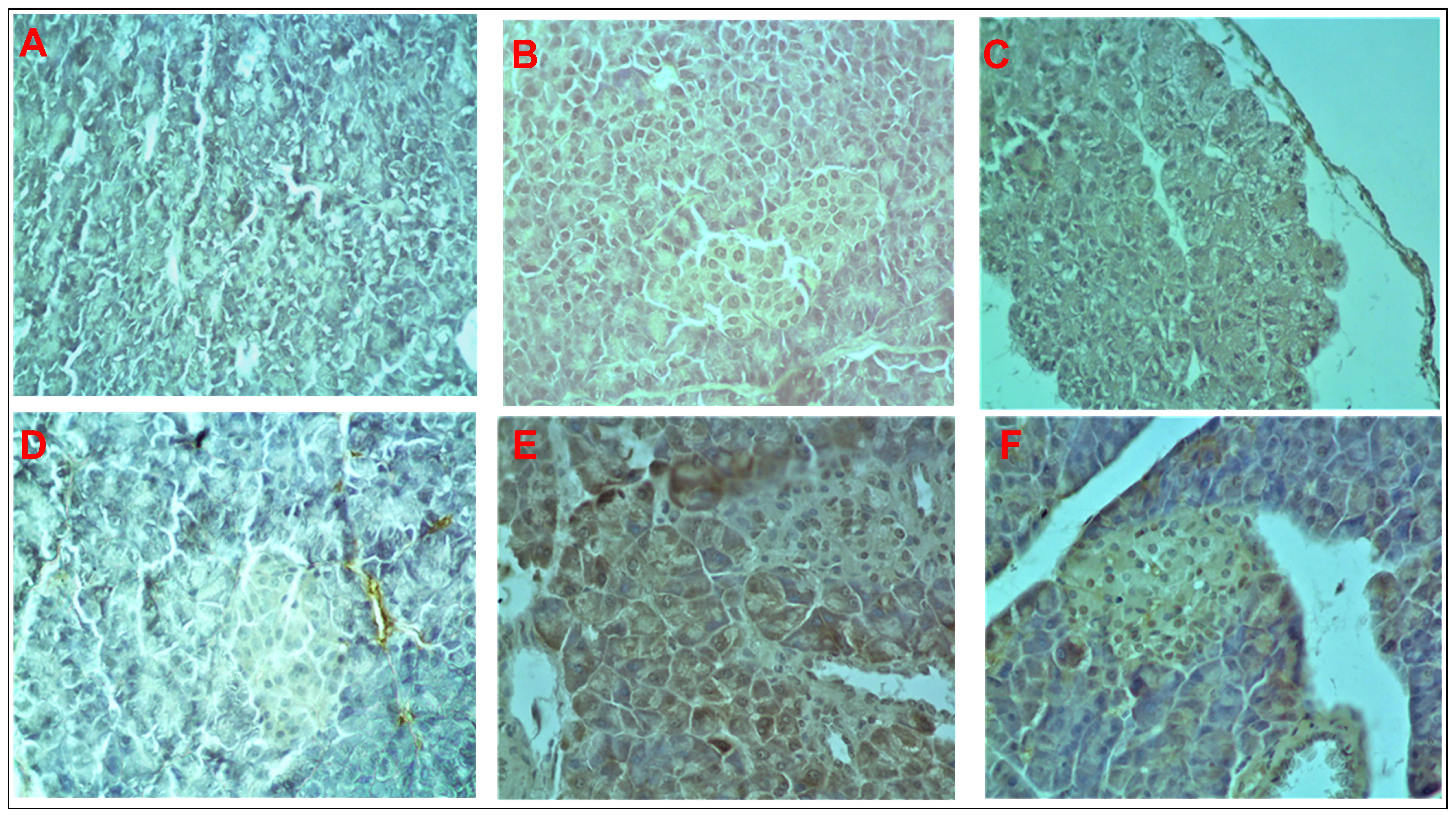
Background: There have been cases of acute pancreatitis brought on by steroids, but identifying it is challenging and necessitates careful monitoring. However, according to new research, 3–5% of all cases of illness may be caused by drug-induced acute pancreatitis, making it the third most common cause. Objective: Evaluation of the effect of hydrocortisone injections on pancreatic structure immunohistochemically using BAX and eNOS markers. Methods: White New Zealand female rabbits weighing between 1.2 and 1.5 kg were used, and they were given free access to food. The rabbits were split into six groups, with five animals in each group receiving intramuscular hydrocortisone injections for 14 and 21 days, respectively, at a dose of 5 mg and 20 mg/kg for short and long durations, and two control groups. Results: There was an increase in weight in both long-duration groups (GL1 and GL2) after week 2 of injection when compared to both control and short-duration groups. There was a highly statistical difference in the expression of BAX in both short- and long-duration groups compared to the control group, and there was also a decrease in the expression of BAX when duration increased. Similarly, there was a highly statistical difference in the expression of eNOS in both the GS and GL groups when compared to the control group. Conclusions: The pancreas can be injured by high and low doses of hydrocortisone if used for more than 2 weeks.
Downloads
References
Alam MM, Okazaki K, Nguyen LTT, Ota N, Kitamura H, Murakami S, et al. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J Biol Chem. 2017;292(18):7519–7530. doi: 10.1074/jbc.M116.773960. DOI: https://doi.org/10.1074/jbc.M116.773960
Wang M, Jiang Z, Liang H. Glucocorticoids in acute pancreatitis: a propensity scores matching analysis. BMC Gastroenterol. 2021;21:331. doi: 10.1186/s12876-021-01907-1. DOI: https://doi.org/10.1186/s12876-021-01907-1
Chan ED, Chan MM, Chan MM, Marik PE. Use of glucocorticoids in the critical care setting: Science and clinical evidence. Pharmacol Ther. 2020;206:107428. doi: 10.1016/j.pharmthera.2019.107428. DOI: https://doi.org/10.1016/j.pharmthera.2019.107428
Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. DOI: https://doi.org/10.1007/s00134-015-4095-4
Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. DOI: https://doi.org/10.1038/d41586-020-01824-5
Wang K, Tan F, Zhou R, Liu D, Ni Z, Liu J, et al. Therapeutic response to corticosteroids in a critically ill patient with COVID-19: A case report. Medicine. 2020;99(31):e21597. doi: 10.1097/MD.0000000000021597. DOI: https://doi.org/10.1097/MD.0000000000021597
Ataallah B, Abdulrahman M, Al-Zakhari R, Buttar BS, Nabeel S. Steroid-induced pancreatitis: A challenging diagnosis. Cureus. 2020;12(7):e8939. doi: 10.7759/cureus.8939. DOI: https://doi.org/10.7759/cureus.8939
Sadr-Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med. 2013;173:444–449. doi: 10.1001/jamainternmed.2013.2737. DOI: https://doi.org/10.1001/jamainternmed.2013.2737
Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294-298. doi: 10.1002/acr.21796. DOI: https://doi.org/10.1002/acr.21796
Makol A, Petri M. Pancreatitis in systemic lupus erythematosus: frequency and associated factors—a review of the Hopkins Lupus Cohort. J Rheumatol. 2010;37(2):341-345. doi: 10.3899/jrheum.090829. DOI: https://doi.org/10.3899/jrheum.090829
Chawla S, Atten MJ, Attar BM. Acute pancreatitis as a rare initial manifestation of Wegener’s granulomatosis: a case based review of literature. JOP. 2011;12(2):167-169. PMID: 21386646.
Kandil E, Lin YY, Bluth MH, Zhang H, Levi G, Zenilman ME. Dexamethasone mediates protection against acute pancreatitis via upregulation of pancreatitis-associated proteins. World J Gastroenterol. 2006;12(42):6806-6811. doi: 10.3748/wjg.v12.i42.6806.
Zhang XP, Chen L, Hu QF, Tian H, Xu RJ, Wang ZW, et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13(41):5506-5511. doi: 10.3748/wjg.v13.i41.5506. DOI: https://doi.org/10.3748/wjg.v13.i41.5506
Sugiyama Y, Kato S, Abe M, Mitsufuji S, Takeuchi K. Different effects of dexamethasone and the nitric oxide synthase inhibitor L-NAME on caerulein-induced rat acute pancreatitis, depending on the severity. Inflammopharmacology. 2005;13:291–301. doi: 10.1163/156856005774423728. DOI: https://doi.org/10.1163/156856005774423728
Paszt A, Takács T, Rakonczay Z, Kaszaki J, Wolfard A, Tiszlavicz L, et al. The role of the glucocorticoid-dependent mechanism in the progression of sodium taurocholate-induced acute pancreatitis in the rat. Pancreas. 2004;29:75-82. doi: 10.1097/00006676-200407000-00059. DOI: https://doi.org/10.1097/00006676-200407000-00059
Noel KI. Hepatic tissues under the effect of dexamethasone: Histological study, dose and duration related changes. Iraqi J Med Sci. 2013;11(2):113-118.
Wang ZF, Pan CE, Lu Y, Liu SG, Zhang GJ, Zhang XB. The role of inflammatory mediators in severe acute pancreatitis and regulation of glucocorticoids. Hepatobiliary Pancreat Dis Int. 2003;2:458-462. PMID: 14599960.
Liu JS, Wei XG, Fu J, Liu J, Yuan YZ, et al. Stady of the relationship among endothelin, nitric oxide, oxgen free radical and acute pancreatitis. Zhongguo Yishi Zazhi. 2003;5:28–29.
Dong R, Wang ZF, LV Y, Ma QJ. Treatment of severe acute pancreatitis with large dosage of dexamethasone in the earlier time. Gandan Waike Zazhi. 2005;13:58–60.
Wang ZF, Liu C, Lu Y, Dong R, Xu J, Yu L, et al. Dexamethasone and dextran 40 treatment of 32 patients with severe acute pancreatitis. World J Gastroenterol. 2004;10:1333-1336. doi: 10.3748/wjg.v10.i9.1333. DOI: https://doi.org/10.3748/wjg.v10.i9.1333
Al-Kaabi M, Noel K, Al-Rubai A. Evaluation of immunohistochemical expression of stem cell markers (NANOG and CD133) in normal, hyperplastic, and malignant endometrium. J Med Life. 2022;15(1):117-123. doi: 10.25122/jml-2021-0206. DOI: https://doi.org/10.25122/jml-2021-0206
Kandil E, Lin Y, Levy G, Bluth M, Zenilman M. Dexamethasone mediates protection against acute pancreatitis in rats via upregulation of pancreatitis of pancreatitis-associated protein (PAP). Gastroenterology. 2004;126:A781. doi: 10.3748/wjg.v12.i42.6806. DOI: https://doi.org/10.3748/wjg.v12.i42.6806
Campbell PD, Weinberg A, Chee J, Mariana L, Ayala R, Hawthorne WJ, et al. Expression of pro- and antiapoptotic molecules of the Bcl-2 family in human islets post isolation. Cell Transplant. 2012;21(1):49-60. doi: 10.3727/096368911X566262. DOI: https://doi.org/10.3727/096368911X566262
Frossard JL, Quadri R, Hadengue A, Morel P, Pastor CM. Endothelial nitric oxide synthase regulation is altered in pancreas from cirrhotic rats. World J Gastroenterol. 2006;12(2):228-233. doi: 10.3748/wjg.v12.i2.228. DOI: https://doi.org/10.3748/wjg.v12.i2.228
Nikolaou N, Hodson L, Tomlinson JW. The role of 5-reduction in physiology and metabolic disease: evidence from cellular, pre-clinical and human studies. J Steroid Biochem Mol Biol. 2021;207:105808. doi: 10.1016/j.jsbmb.2021.105808. DOI: https://doi.org/10.1016/j.jsbmb.2021.105808
Al-Mosawi RH, Alebadi NAM. Usage of corticosteroids as therapeutic agents in diseases. Med J Babylon. 2023;20:447-450. doi: 10.4103/MJBL.MJBL_391_23.
Matthes H, Kaiser A, Stier U, Riecken EO, Rosewicz S. Glucocorticoid receptor gene expression in the exocrine and endocrine rat pancreas. Endocrinology. 1994;135(1):476-479. doi: 10.1210/endo.135.1.8013388. DOI: https://doi.org/10.1210/endo.135.1.8013388
Hameed AF, Noel KI, Akkila SS. Placental angiogenesis, IUGR & CMV awareness in Iraqi women. Curr Issues Pharm Med Sci. 2022;35(3):147-151. doi: 10.2478/cipms-2022-0027. DOI: https://doi.org/10.2478/cipms-2022-0027
Noel KI, Ibraheem MM, Ahmed BS, Hameed AF, Khamees NH, Akkila SS. Expression of OCT4 stem cell marker in benign prostatic hyperplasia and normal tissue around the prostatic carcinoma in a sample of Iraqi patients. Egypt J Histol. 2020;43(1):245-254. doi: 10.21608/EJH.2019.13791.1130. DOI: https://doi.org/10.21608/ejh.2019.13791.1130
Noel KI, Ibraheem MM, Ahmed BS, Hameed AF, Khamees NH, Akkila SS. CD133 and CD166 expression predicting the possibility of prostatic cancer development in cases of BPH. Biomed Pharmacol J. 2019;12(3):1403-1416. doi: 10.13005/bpj/1769. DOI: https://doi.org/10.13005/bpj/1769
Bannon CAM, Border D, Hanson P, Hattersley J, Weickert MO, Grossman A, et al. Early metabolic benefits of switching hydrocortisone to modified release hydrocortisone in adult adrenal insufficiency. Front Endocrinol (Lausanne). 2021;12:641247. doi: 10.3389/fendo.2021.641247. DOI: https://doi.org/10.3389/fendo.2021.641247
Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3): e219. doi: 10.1038/emm.2016.6. DOI: https://doi.org/10.1038/emm.2016.6
Nomoto H, Gurlo T, Rosenberger M, Girgis MD, Dry S, Butler PC. Low grade islet but marked exocrine pancreas inflammation in an adult with autoimmune pre-diabetes. Case Rep Endocrinol. 2019;2019:5863569. doi: 10.1155/2019/5863569. DOI: https://doi.org/10.1155/2019/5863569
Guo L, Lichten LA, Ryu MS, Liuzzi JP, Wang F, Cousins RJ. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci U S A. 2010;107(7):2818-2823. doi: 10.1073/pnas.0914941107. DOI: https://doi.org/10.1073/pnas.0914941107
Zhang J, Ge P, Liu J, Luo Y, Guo H, Zhang G, et al. Glucocorticoid treatment in acute respiratory distress syndrome: An overview on mechanistic insights and clinical benefit. Int J Mol Sci. 2023;24(15):12138. doi: 10.3390/ijms241512138. DOI: https://doi.org/10.3390/ijms241512138
Vegiopoulos A, Herzig S, Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275(1-2):43-61. doi: 10.1016/j.mce.2007.05.015. DOI: https://doi.org/10.1016/j.mce.2007.05.015
Cutroneo KR, Sterling KM. How do glucocorticoids compare to oligo decoys as inhibitors of collagen synthesis and potential toxicity of these therapeutics? J Cell Biochem. 2004;92:6-15. doi: 10.1002/jcb.20030. DOI: https://doi.org/10.1002/jcb.20030
Esguerra JLS, Ofori JK, Nagao M, Shuto Y, Karagiannopoulos A, Fadista J, et al. Glucocorticoid induces human beta cell dysfunction by involving riborepressor GAS5 LincRNA. Mol Metab. 2020;32:160-167. doi: 10.1016/j.molmet.2019.12.012. DOI: https://doi.org/10.1016/j.molmet.2019.12.012
Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853-859. doi: 10.1097/01.lab.0000074918.31303.5a. DOI: https://doi.org/10.1097/01.LAB.0000074918.31303.5A
Saleh A, Srinivasula SM, Acharya S, Fishel R, Alnemri ES. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J Biol Chem. 1999;274:17941–17945. doi: 10.1074/jbc.274.25.17941. DOI: https://doi.org/10.1074/jbc.274.25.17941
Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1-30. doi: 10.1016/S0079-6123(10)82001-1. DOI: https://doi.org/10.1016/S0079-6123(10)82001-1
Herr I, Gassler N, Friess H, Buchler MW. Regulation of differential pro- and anti-apoptotic signaling by glucocorticoids. Apoptosis. 2007;12:271-291. doi: 10.1007/s10495-006-0624-5. DOI: https://doi.org/10.1007/s10495-006-0624-5
Gruver-Yates AL, Cidlowski JA. Tissue-specific actions of glucocorticoids on apoptosis: a double-edged sword. Cells. 2013;2(2):202-223. doi: 10.3390/cells2020202. DOI: https://doi.org/10.3390/cells2020202
Hashish HA. Effect of metformin on Bax expression in pancreas and testis of normoglycemic albino rat: An immunohistochemical study. Indian J Clin Anat Physiol. 2020;7(2):134-141. doi: 10.18231/j.ijcap.2020.028. DOI: https://doi.org/10.18231/j.ijcap.2020.028
Shah V, Cao S, Hendrickson H, Yao J, Katusic ZS. Regulation of hepatic eNOS by caveolin and calmodulin after bile duct ligation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1209–G1216. doi: 10.1152/ajpgi.2001.280.6.G1209. DOI: https://doi.org/10.1152/ajpgi.2001.280.6.G1209
Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459(6):793-806. doi: 10.1007/s00424-009-0767-7. DOI: https://doi.org/10.1007/s00424-009-0767-7
Ahmed TH, Mshimesh BAR, Raoof IB. Effect of nicorandil on endothelial markers and tissue remodeling in pulmonary arterial hypertension model of male rats. Al-Rafidain J Med Sci. 2023;5(1S):S87-93. doi: 10.54133/ajms.v5i1S.334. DOI: https://doi.org/10.54133/ajms.v5i1S.334
Lei Ying, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67(4):1407-1410. doi: 10.1158/0008-5472.CAN-06-2149. DOI: https://doi.org/10.1158/0008-5472.CAN-06-2149
Araujo JEDS, Miguel-Dos-Santos R, Macedo FN, Cunha PS, Fontes MT, Murata GM, et al. Effects of high doses of glucocorticoids on insulin-mediated vasodilation in the mesenteric artery of rats. PLoS One. 2020;15(3):e0230514. doi: 10.1371/journal.pone.0230514. DOI: https://doi.org/10.1371/journal.pone.0230514
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











