Design, Synthesis, Characterization and Preliminary Evaluation of New 1H-benzo[d]imidazole-1yl-derivatives as Acetylcholine Esterase Inhibitors
DOI:
https://doi.org/10.54133/ajms.v7i1.794Keywords:
AchE inhibitors, Benzo[d]imidazole, Characterization, Design, SynthesisAbstract
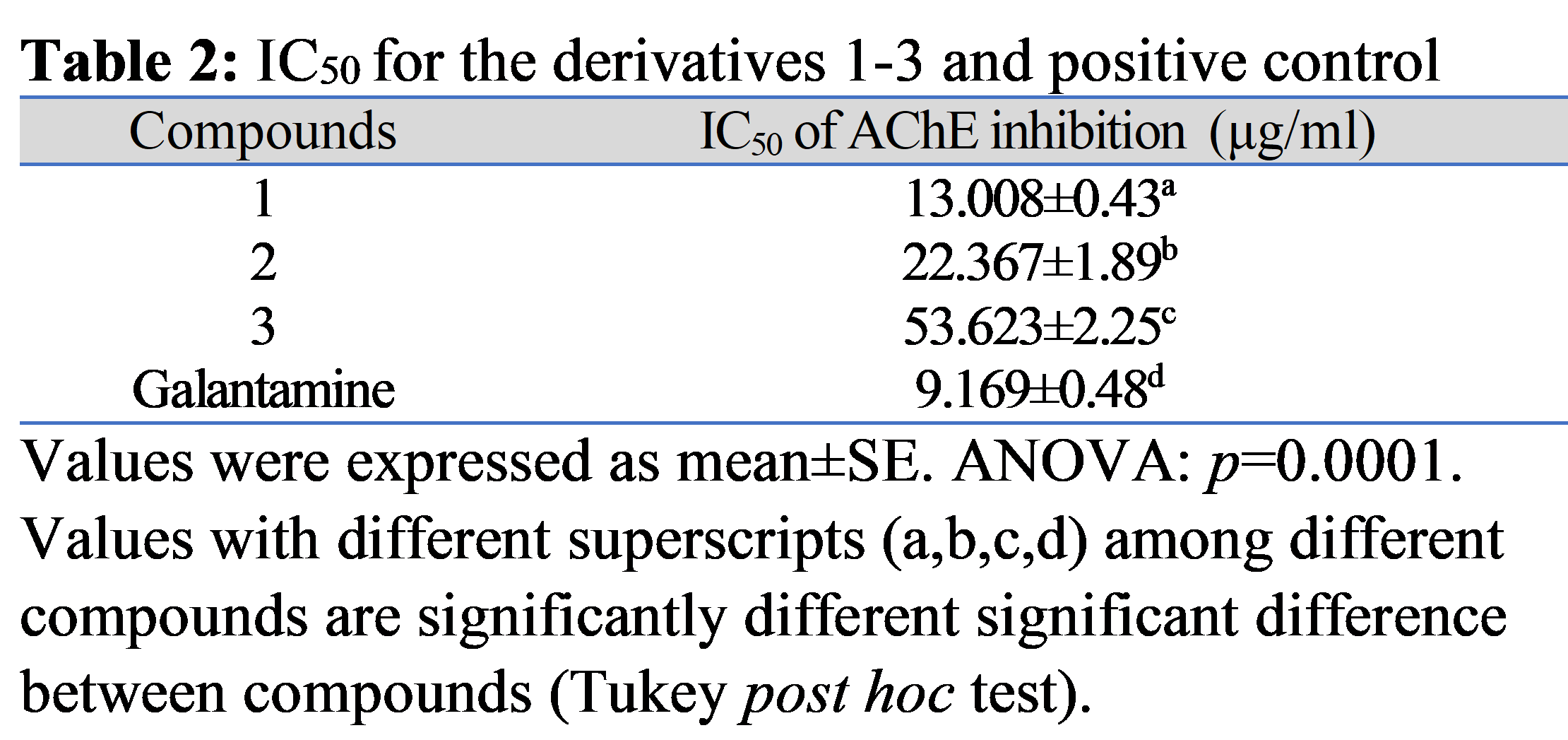
Background: Alzheimer disease (AD) is the most common type of dementia, which is still a problem that everyone must deal with. In a continuous effort to find effective treatments, the new candidates for AD therapy have the capacity to scavenge excessive levels of free radicals and inhibit acetylcholinesterase (AChE). Objectives: This study focuses on synthesizing and biologically evaluating novel hybrid compounds (1-3) as acetylcholine esterase inhibitors. Methods: The benzimidazole has been added and then coupled with coumaric acid, cinnamic acid, and lipoic acid as conjugates, which are expected to have dual action as acetylcholinesterase inhibitors and antioxidants. The synthesis of benzimidazole derivatives (1-3) was accomplished and then characterized using 1H-NMR and elemental analysis. Additionally, their characteristics were assessed in vitro against the AChE enzyme. Results: The new compounds produced a potent inhibitory activity that may serve as a lead molecule for the synthesis of novel anti-AD molecules. Compound-1 has an inhibition percentage that is close to that of the authorized medication galantamine (95.386%), whereas compound-3 has the lowest inhibition percentage (88.647%). Conclusions: A very good yield was achieved during the synthesis of the benzimidazole derivatives (1-3) from the starting material. They can serve as potential candidates for acetylcholine esterase inhibitors.
Downloads
References
Türkan F. Investigation of the toxicological and inhibitory effects of some benzimidazole agents on acetylcholinesterase and butyrylcholinesterase enzymes. Arch Physiol Biochem. 2021;127(2):97-101. doi: 10.1080/13813455.2019.1618341.
Maness EB, Burk JA, McKenna JT, Schiffino FL, Strecker RE, McCoy JG. Role of the locus coeruleus and basal forebrain in arousal and attention. Brain Res Bull. 2022;188:47-58. doi: 10.1016/j.brainresbull.2022.07.014.
Chen ZR, Huang JB, Yang SL, Hong FF. Role of cholinergic signaling in Alzheimer’s disease. Molecules. 2022;27(6):1816. doi: 10.3390/molecules27061816.
Bosia M, Cook F, Bigai G, Martini F, Fregna L, Leave C, et al, (Eds.), Organic mental disorders and psychiatric issues in the elderly. In: Fundamentals of Psychiatry for Health Care Professionals, Springer; 2022, p. 297-331. doi: 10.1111/jar.13217.
Melgarejo da Rosa M, Cassimiro de Amorim L, Victor de Oliveira Alves J, Fidélis da Silva Aguiar I, Granja da Silva Oliveira F, Vanusa da Silva M. The promising role of natural products in Alzheimer's disease. Brain Disord. 2022;7:100049. doi: 10.1016/j.dscb.2022.100049.
Sharifi-Rad J, Rapposelli S, Sestito S, Herrera-Bravo J, Arancibia-Diaz A, Salazar LA, et al. Multi-target mechanisms of phytochemicals in Alzheimer’s disease: Effects on oxidative stress, neuroinflammation and protein aggregation. J Personalized Med. 2022;12(9):1515. doi: 10.3390/jpm12091515.
Rekha K, Neerja V, Shailja S, Taha A, Mohannad A, Alexander N, et al. Ameliorative effects of phytomedicines on Alzheimer’s patients. Curr Alzheimer Res. 2022;19(6):420-439. doi: 10.2174/1567205019666220610155608.
Anurag Tk, Amit K, Rajnish K, Taher D. Osteric binding sites of Aβ peptides on the acetylcholine synthesizing enzyme ChAT as deduced by in silico molecular modeling. Int J Mol Sci. 2022;23(11):6073. doi: 10.3390/ijms23116073.
Garrett D, Naoya T, Kazuya T, Shun-ichi I, Sotiris S, Masaaki F. Structure of gas phase monohydrated nicotine: Implications for nicotine’s native structure in the acetylcholine binding protein. J Am Chem Soc. 2022;144(37):16698-16702. doi: 10.1021/jacs.2c04064.
Javad S, Simona R, Simona S, Jesús H, Alejandra A, Luis A, et al. Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2022:116742. doi: 10.1016/j.bmc.2022.116742.
Shohag S, Akhter S, Islam S, Sarker T, Sifat MK, Rahman MM, et al. Perspectives on the molecular mediators of oxidative stress and antioxidant strategies in the context of neuroprotection and neurolongevity: An extensive review. Oxid Med Cell Longev. 2022;2022:7743705. doi: 10.1155/2022/7743705.
Tarana U, Rampratap M, Muste H, Pawan K, Asim A. Recent updates in development of small molecules as potential clinical candidates for Alzheimer’s disease: A review. Chem Biol Drug Design. 2022;100(5):674-681. doi: 10.1111/cbdd.14133.
Srinivas R, Salwa S, Navya A, Shirleen M, Lalit K. Recent advances in nanoformulation development of Ritonavir, a key protease inhibitor used in the treatment of HIV-AIDS. Expert Opin Drug Deliv. 2022;19(9):1133-1148. doi: 10.1080/17425247.2022.2121817.
Xing N, Meng X, Wang S. Isobavachalcone: A comprehensive review of its plant sources, pharmacokinetics, toxicity, pharmacological activities and related molecular mechanisms. Phytother Res. 2022;36(8):3120-3142. doi: 10.1002/ptr.7520.
Villablanca E, Selin K, Hedin C. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression. Nature Rev Gastroenterol Hepatol. 2022:1-15. doi: 10.1038/s41575-022-00604-y.
Bischoff S. European guideline on obesity care in patients with gastrointestinal and liver diseases Joint ESPEN/UEG guideline. Clin Nutr. 2022;41(10):2364-2405. doi: 10.1016/j.clnu.2022.07.003.
Nutho B, Yanarojana S, Supavilai P, Structural dynamics and susceptibility of anti-Alzheimer’s drugs donepezil and galantamine against human acetylcholinesterase.
Kumar A, Nimsarkar P, Singh S, Systems pharmacology aiding benzimidazole scaffold as potential lead compounds against leishmaniasis for functional therapeutics. Life Sci. 2022:120960. doi: 10.1016/j.lfs.2022.120960.
Leggio A, Belsito EL, De Luca G, Di Gioia ML, Leotta V, Romio E, et al. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. Rsc Advances. 2016;6(41):34468-34475.
Ömer Ş, Ümmühan Ö, Nurgül S, Şevki A, Zeynel S, Synthesis, characterization, molecular docking and in vitro screening of new metal complexes with coumarin Schiff base as anticholine esterase and antipancreatic cholesterol esterase agents. J Biomol Struct Dyn. 2022;40(10):4460-4474. doi:10.1080/07391102.2020.1858163.
Ali-Shtayeh MS, Jamous RM, Zaitoun SY, Qasem IB, In-vitro screening of acetylcholinesterase inhibitory activity of extracts from Palestinian indigenous flora in relation to the treatment of Alzheimer’s disease. Funct Food Health Dis. 2014;4(9):381-400. doi: 10.31989/ffhd. v4i9.149.
Tan L, Guo S, Ma F, Chang C, Gómez-Betancur I, In vitro inhibition of acetylcholinesterase, alphaglucosidase, and xanthine oxidase by bacteria extracts from coral reef in Hainan, South China Sea. J Marine Sci Engineer. 2018;6(2):33. doi: 10.3390/jmse6020033.
Ferreira J, Santos S, Pereira H, In vitro screening for acetylcholinesterase inhibition and antioxidant activity of Quercus suber cork and corkback extracts. Evidence-Based Complement Altern Med. 2020; 2020:3825629. doi: 10.1155/2020/3825629.
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











