Synthesis, Characterization, Molecular Docking, ADMET Study, and Antimicrobial Evaluation of New Mannich Bases of Isatin–Thiazole Imine Bases
DOI:
https://doi.org/10.54133/ajms.v6i2.835Keywords:
2-aminothiazole, Antimicrobial activity, Isatin, Mannich basesAbstract
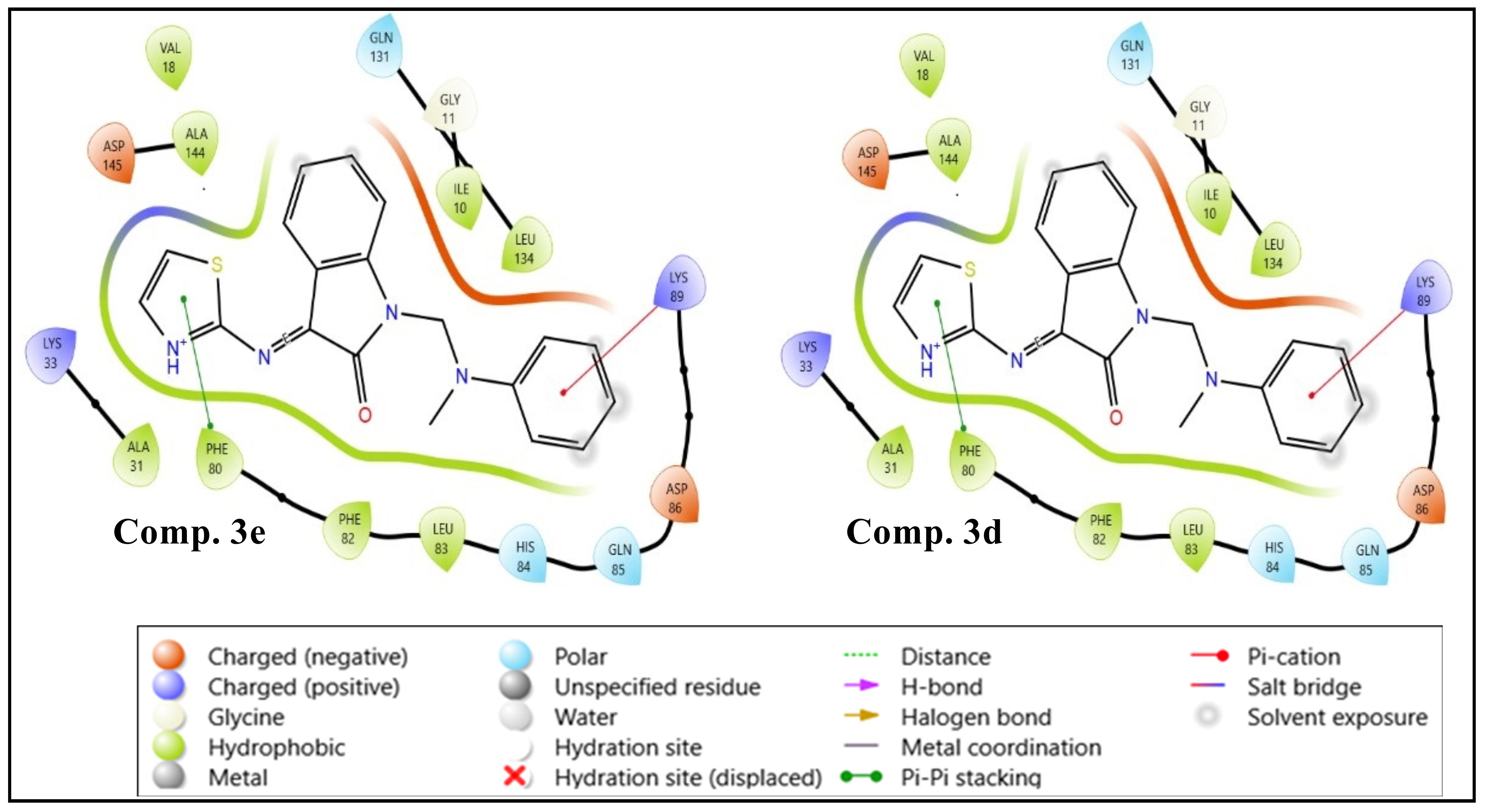
Background: The isatin molecule is present in many natural substances, including plants and animals, and is used to prepare compounds with various biological activities. Objectives: To synthesize a new series of isatin derivatives with the expectation that they will have antimicrobial activity. Methods: Thiazole Schiff bases were synthesized from various Mannich bases of isatin to evaluate their antimicrobial properties. Initially, Mannich bases (2a–e) were synthesized by reacting isatin with formaldehyde and different secondary amines. Subsequently, they were treated with 2-aminothiazole to yield the final compounds (3a–e). Spectroscopic characterization was done via FT-IR and 1H-NMR. The antimicrobial screening was conducted on all derivatives. Molecular docking and ADMET analysis were performed on the final compounds, comparing them with standard drugs (ciprofloxacin and fluconazole). Results: The antimicrobial activity was assessed on two Gram-positive bacteria, Staphylococcus aureus and Bacillus licheniformis; two Gram-negative bacteria, Escherichia coli and Acinetobacter baumannii; and one fungus species, Candida albicans. Molecular docking has recorded higher docking scores for 3d and 3e compared to ciprofloxacin and fluconazole. The virtually active molecules showed an adequate drug-like profile and desired pharmacokinetic properties in the ADMET analysis. Conclusions: Most derivatives displayed significant antimicrobial activity, with compound 3e being the most active, followed by compound 3b. Molecular docking revealed higher scores for compound 3e compared to fluconazole and for compounds 3d and 3e compared to ciprofloxacin. ADMET analysis of compound 3e showed excellent absorption, consistent with its strong GIT absorption.
Downloads
References
Al-Mudhafar MM, Kamoon RA. Synthesis, characterization, and antimicrobial activity of new Schiff’s and Mannich bases of isatin and isatin derivatives. J Glob Pharma Technol. 2020;12(1):529-536.
Chirra S, Shama SN, Pranitha D, Reddy CM, Jupally VR. Study of antimicrobial activity of novel Isatin derivatives. World J Biol Pharm Health Sci. 2023;13(1):039-043. doi: 10.30574/wjbphs.2023.13.1.0285.
Mukhlif MM, Al-Mudhafar MM. Synthesis, characterization, and preliminary antimicrobial evaluation of new Schiff bases and Mannich bases of isatin. Iraqi J Pharm Sci. 2023;32(Suppl.):156-163. doi: 10.31351/vol32issSuppl.pp156-16.
Bogdanov AV, Vazykhova AB, Khasiyatullina NR, Krivolapov DB, Dobrynin AB, Voloshina AD, et al. New N-Mannich bases obtained from isatin and piperazine derivatives: the synthesis and evaluation of antimicrobial activity. Chem Heterocyclic Compound. 2016;52: 25-30. doi: 10.1007/s10593-016-1826-6.
Hassan AS, Morsy NM, Aboulthana WM, Ragab A. Exploring novel derivatives of isatin-based Schiff bases as multi-target agents: design, synthesis, in vitro biological evaluation, and in silico ADMET analysis with molecular modeling simulations. RSC Adv. 2023;13(14):9281-9303. doi: 10.1039/D3RA00297G.
Katarzyna B, Chruscinska EL, Schiff bases –interesting range of applications in various fields of science, CHEMIK. 2014;68(2):129-134. doi: 10.1002/chin.201511346.
Prakash A, Adhikari D. Application of Schiff bases and their metal complexes-A Review. Int J Chem Tech Res. 2011;3(4):1891-1896.
Qin W, Long S, Panunzio M, Biondi S. Schiff bases: a short survey on an evergreen chemistry tool. Molecules. 2013;18(10):12264-12289. doi: 10.3390/molecules181012264.
Mithun R, Biplab D. Chemistry and biological importance of heterocyclic Schiff bases. Int Res J Pure Appl Chem. 2013;3(3):232-249. doi: 10.9734/IRJPAC/2013/3996.
Sahib HA, Mohammed MH. Synthesis and preliminary biological activity evaluation of new N-substituted phthalimide derivatives. Iraqi J Pharm Sci. 2020;29(1):247-252. doi: 10.31351/vol29iss1pp247-25.
Tamer AA, Qassir AJ. Synthesis of acetylenic derivatives of substituted 1, 3, 4-Thiadiazole as antibacterial agents. Iraqi J Pharm Sci. 2019;28(1):124-130. doi: 10.31351/vol28iss1pp124-130.
Raoof SS, Sadiq AS. Mannich bases: Synthesis, pharmacological activity, and applications: A review. Iraqi J Sci. 2022;63(12):5086-5105. doi: 10.24996 /ijs.2022.63.12.1.
Oloyede GK, Willie IE, Adeeko OO. Synthesis of Mannich bases: 2-(3-Phenylamino propionyloxy)- benzoic acid and 3-Phenylamino-1-(2, 4, 6-trimethoxy-phenyl)-propan-1-one, their toxicity, ionization constant, antimicrobial and antioxidant activities. Food Chem. 2014;165:515-521. doi: 10.1016/j.foodchem.2014.05.119.
Pakravan P, Kashanian S, Khodaei MM, Harding FJ. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacol Rep. 2013;65(2):313-335. doi: 10.1016/s1734-1140(13)71007-7.
Chhajed SS, Padwal MS. Antimicrobial evaluation of some novel Schiff and Mannich bases of isatin and its derivatives with quinolin. Int J Chem Tech Res. 2010;2(1):209-213.
Shmidt MS, Reverdito AM, Kremenchuzky L, Perillo IA, Blanco MM. Simple and efficient microwave-assisted N-alkylation of isatin, Molecules. 2008;13(4):831-840. doi: 10.3390/molecules13040831.
Abdulhadi SL, Abdulkadir MQ, Al-Mudhafar MM. The importance of 2-Aminothiazole Schiff bases as antimicrobial and anticancer agents. Al-Mustansiriyah J Sci. 2020;31(3):46-64. doi: 10.23851/mjs.v31i3.865.
Tsuji K, Ishikawa H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg Med Chem Lett. 1994;4:1601-1606.
Karabasanagouda T, Adhikari AV, Dhanwad R, Parameshwarappa G. Synthesis of some new 2-(4Alkylthiophenoxy)-4-Substituted-1, 3Thiazoles as possible anti-inflammatory and antimicrobial agents, Indian J Chem 2008;47b:144-152.
Zhang ZH, Wu HM, Deng SN, Cai XY, Yao Y, et al. Design, synthesis, and anticancer activities of novel 2-Amino-4-phenylthiazole scaffold containing amide moieties. J Chem. 2018;2018:1-8. doi: 10.1155/2018/4301910.
Kumar K, Liu N, Yang D, Na D, Thompson J, Wrischnik LA, et al. Synthesis and antiprotozoal activity of mono-and bis-uracil isatin conjugates against the human pathogen Trichomonas vaginalis. Bioorg Med Chem. 2015;23(16):5190-5197. doi: 10.1016/j.bmc.2015.04.075.
Debnath B, Ganguly S. Synthesis, characterization, and anthelmintic activity of isatin analogs against Pheritima Posthuma. Asian J Pharm Clin Res. 2015;8(5):150-155.
Manan MA, Crouse KA, Tahir MI, Rosli R, How FN, Watkin DJ, et al. Synthesis, characterization and cytotoxic activity of S-benzyldithiocarbazate Schiff bases derived from 5-fluoroisatin, 5-chloroisatin, 5-bromoisatin and their crystal structures. J Chem Crystallogr. 2011;41:1630-41. doi: 10.1007/s10870-011-0151-2.
Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. Il Farmaco. 1999;54(9):624-628. doi: 10.1016/s0014-827x(99)00075-0.
Karki SS, Kulkarni AA, Kumar S, Veliyath SK, De Clercq E, Balzarini J. Synthesis and biological evaluation of 2-(5-substituted-1-((diethylamino) methyl)-2-oxoindolin-3-ylidene)-N-substituted-hydrazine carbothioamides. Med Chem Res. 2013;22:2014–2022. doi: 10.1007/s00044-012-0184-x.
Prakash CR, Raja SU, Saravanan G. Synthesis, analgesic, anti-inflammatory and in vitro antimicrobial studies of some novel Schiff and Mannich base of 5-substituted isatin derivatives. Int J Pharmacy Pharm Sci. 2014;6(10):160-166.
Yu S, Wang N, Chai X, Wang B, Cui H, Zhao Q, et al. Synthesis and antifungal activity of the novel triazole derivatives containing 1, 2, 3-triazole fragment. Arch Pharm Res. 2013;36:1215-1222. doi:10.3390/molecules190811333.
Siwek A, Stączek P, Wujec M, Stefańska J, Kosikowska U, Malm A, et al. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J Mol Model. 2011;17:2297-2303. doi: 10.1007/s00894-010-0889-z.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Analysis. 2016;6(2):71-79.
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739-1749. doi: 10.1021/jm0306430.
Madhavi Sastry G, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Computer-Aided Mol Desig. 2013;27:221-34. doi: 10.1007/s10822-013-9644-8.
Ghabbour HA, Qabeel MM, Eldehna WM, Al-Dhfyan A, Abdel-Aziz HA. Design, synthesis, and molecular docking of 1-(1-(4-chlorophenyl)-2-(phenylsulfonyl) ethylidene)-2-phenylhydrazine as a potent nonazole anticandidal agent. J Chem. 2014;2014. doi: 10.1155/2014/154357.
Hasan Y, Al-hamashi A. Identification of selisistat derivatives as SIRT1-3 inhibitors by in silico virtual screening. Turk Computat Theor Chem. 2023;8(2):1-1. doi: 10.33435/tcandtc.1224592.
Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, et al. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput. 2016;12(1):281-296. doi: 10.1021/acs.jctc.5b00864.
Chidambaram K. Identification of BACE-1 inhibitors against Alzheimer’s disease through E-pharmacophore-based virtual screening and molecular dynamics simulation studies: An in silco approach. Life. 2023;13(4):952. doi: 10.3390/life13040952.
Al-Kadhimi AA, Al-Azzawi NK, Khalaf AI. Facile synthesis of Schiff and Mannich bases of isatin derivatives. J Chem Biol Phys Sci. 2015;5(3):2338-2349.
de Almeida CG, Garbois GD, Amaral LM, Diniz CC, Le Hyaric M. Relationship between structure and antibacterial activity of lipophilic N-acyldiamines. Biomed Pharmacother. 2010;64(4):287-290. doi: 10.1016/j.biopha.2009.09.013.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











