Preparation and Characterization of Febuxostat Nanosuspension as Fast Dissolving Oral Film
DOI:
https://doi.org/10.54133/ajms.v6i2.873Keywords:
Febuxostat, Fast-dissolving oral films, Nanosuspension, Solvent casting methodAbstract
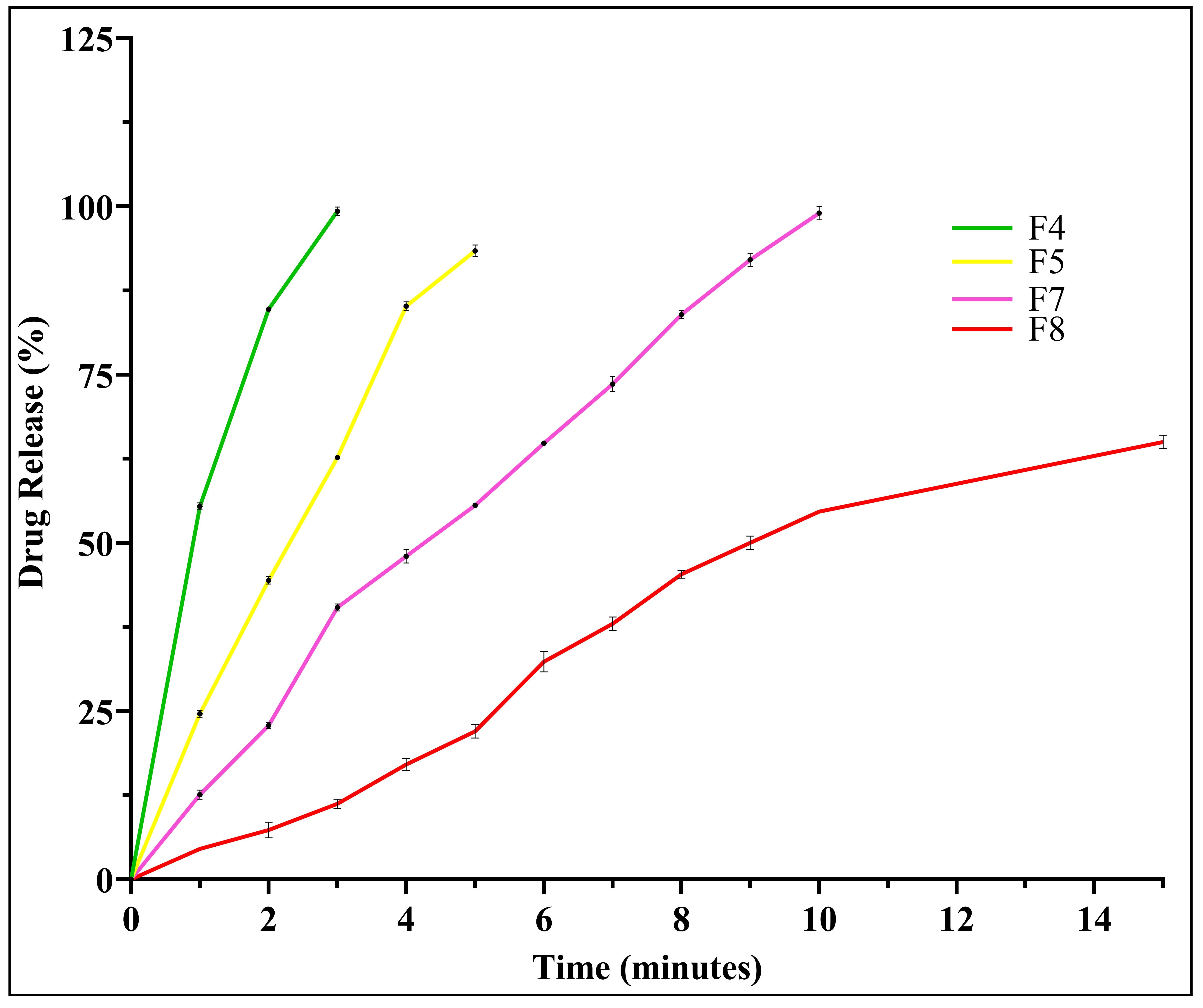
Background: Quickly dissolved oral films are a widely accepted method of delivering drugs and help patients adhere to treatment regimens. Nanosuspensions (NS) are colloidal dispersions of drug particles with a submicron size, and their large surface area enhances the solubility and dissolution of low-water-soluble drugs. Febuxostat (FXT) is a non-purine xanthine oxidase inhibitor with a low dissolution rate that limits its absorption. Objective: To develop fast-dissolving oral films (FDOFs) containing FXT NS and convert NS into solid dosage forms to ease administration and accelerate drug release. Methods: FXT NS was prepared using Soluplus as a stabilizer and Tween80 as a co-stabilizer through an anti-solvent precipitation technique. We prepared FDOFs using a solvent casting method, utilizing hydrophilic polymers like pullulan, polyvinyl alcohol (PVA), gelatin, and plasticizers like polyethylene glycol (PEG400) and glycerin. The study assessed the film's thickness, weight, folding endurance, drug content, disintegration time, and drug release. We validated the drug's compatibility using FTIR, and conducted a crystallinity study using DSC and X-ray powder diffraction. Results: F4 was the optimized formula prepared using PVA and PEG400. In just three minutes, the F4 dissolution rate increased significantly (99.63% vs. 11.23%) compared to the FXT ordinary film. Also, it had good mechanical properties. Conclusions: FXT NS were successfully loaded into FDOFs with accepted properties.
Downloads
References
Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. doi: 10.1038/s41572-019-0115-y.
Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(3S):S11-S16. doi: 10.1016/j.semarthrit.2020.04.008.
Xie H, Hu N, Pan T, Wu JC, Yu M, Wang DC. Effectiveness and safety of different doses of febuxostat compared with allopurinol in treating hyperuricemia: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. 2023;24(1):79. doi: 10.1186/s40360-023-00723-5.
Patel J, Jagia M, Bansal AK, Patel S. Characterization and thermodynamic relationship of three polymorphs of a xanthine oxidase inhibitor, febuxostat. J Pharm Sci. 2015;104(11):3722–2730. doi: 10.1002/jps.24570.
Zhang XR, Zhang L. Simultaneous enhancements of solubility and dissolution rate of poorly water-soluble febuxostat via salts. J Mol Struct. 2017;1137:328–334. doi: 10.1016/j.molstruc.2017.02.052.
Tang J, Bao J, Shi X, Sheng X, Su W. Preparation, optimization, and in vitro–in vivo evaluation of febuxostat ternary solid dispersion. J Microencapsul. 2018;35(5):454-466. doi: 10.1080/02652048.2018.1526339.
Fuhrmann K, Gauthier MA, Leroux JC. Crosslinkable polymers for nanocrystal stabilization. J Control Release. 2010;148(1):e12–13. doi: 10.1016/j.jconrel.2010.07.006.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. doi: 10.1038/nrd1494.
Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Adv Drug Deliv Rev. 2001;47(1):3–19. doi: 10.1016/S0169-409X(00)00118-6.
Rajab NA, Jassim ZE, Hameed AM. Preparation and characterization of lacidipine as an oral fast-dissolving film. Drug Invent Today. 2018;10(1):321–326.
Kathpalia H, Gupte A. An introduction to fast dissolving oral thin film drug delivery systems: a review. Curr Drug Deliv. 2013;10(6):667-684. doi: 10.2174/156720181006131125150249.
Pandit Shubham R, Shendge Raosaheb S, PulateAniket J, Patil Suresh D, PathareVaishnavi BPAS. Review on: Mouth dissolving film. Int J Pharm Res Appl. 2021;6(3):370–378. doi: 10.35629/7781-0603370378.
Gavaskar B, Kumar SV, Sharan G, Madhusudan Rao Y. Overview on fast dissolving films. Int J Pharm Pharm Sci. 2010;2(Suppl. 3):29–33.
Patel AR, Prajapati DS, Raval JA. Fast dissolving films (FDFs) as a newer venture in fast dissolving dosage forms. Int J Drug Dev Res. 2010;2(2):232–246.
Lu S, Yu P, He JH, Shuang ZS, Xia YL, Zhang WL, et al. Enhanced dissolution and oral bioavailability of lurasidone hydrochloride nanosuspensions prepared by antisolvent precipitation–ultrasonication method. RSC Adv. 2016;6(54):49052–49059 .doi: 10.1039/C6RA08392G.
Kadhim ZJ, Rajab NA. Formulation and characterization of glibenclamide nanoparticles as an oral film. Int J Drug Deliv Technol. 2022;12(1):387–394. doi: 10.25258/ijddt.12.1.70.
Mathew A, Jacob S, Shyma MS. Nanosuspension loaded oral films: A breakthrough approach. J Pharm Sci Res. 2020;12(8):1012–1017.
Kumar S, Doddayya H. Fabrication and evaluation of mouth-dissolving films of domperidone. Int J Curr Pharm Res. 2023;15(2):36–43. doi: 10.22159/ijcpr.2023v15i2.2088.
Bharti K, Mittal P, Mishra B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J Drug Deliv Sci Technol. 2019;49:420–432. doi: 10.1016/j.jddst.2018.12.013.
Saxena S, Patel A, Kumar Jain SK. Formulation and evaluation of mouth dissolving film of antihypertensive agent Formulation and evaluation of mouth dissolving film of antihypertensive agent. World J Adv Res Rev. 2022;16(2):1107–1116. doi:10.30574/wjarr.2022.16.2.1251.
Bala R, Khanna S, Pawar P, Arora S. Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig. 2013;3(2):67. doi: 10.4103%2F2230-973X.114897.
Sajayan K, Swathy KK, Sarath Chandran C, Jafna MC, Nair RS, Sourav K, et al. Development and evaluation of fast dissolving oral films of mefenamic acid for the management of fever. Indian J Pharm Educ Res. 2023;57(1):s41–51. doi: 10.5530/ijper.57.1s.6.
Jassim ZE, Mohammed MF, Sadeq ZA. Formulation and evaluation of fast dissolving film of lornoxicam. Asian J Pharm Clin Res. 2018;11(9):217. doi: 10.22159/ajpcr.2018.v11i9.27098.
Shamma R, Elkasabgy N. Design of freeze-dried Soluplus/polyvinyl alcohol-based film for the oral delivery of an insoluble drug for the pediatric use. Drug Deliv. 2016;23(2):489–499. doi:10.3109/10717544.2014.921944.
Zhang L, Aloia M, Pielecha-Safira B, Lin H, Rajai PM, et al. Impact of superdisintegrants and film thickness on disintegration time of strip films loaded with poorly water-soluble drug microparticles. J Pharm Sci. 2018;107(8):2107-2118. doi: 10.1016/j.xphs.2018.04.006.
Ahuja BK, Jena SK, Paidi SK, Bagri S, Suresh S. Formulation, optimization and in vitro-in vivo evaluation of febuxostat nanosuspension. Int J Pharm. 2015;478(2):540–552. doi: 10.1016/j.ijpharm.2014.12.003.
Xu T, Li H, Xia Y, Ding S, Yang Q, Yang G. Three-Dimensional-Printed Oral Films Based on LCD: Influence Factors of the Film Printability and Received Qualities. Pharmaceutics. 2023;15(3):758. doi: 10.3390/pharmaceutics15030758.
Jabir SA, Sulaiman HT. Preparation and characterization of lafutidine as immediate release oral strip using different type of water-soluble polymer. Int J Appl Pharm. 2018;10(5):249–260. doi: 10.22159/ijap.2018v10i5.28292.
Kini A, Patel SB. Phase behavior, intermolecular interaction, and solid-state characterization of amorphous solid dispersion of Febuxostat. Pharm Dev Technol. 2017;22(1):45–57. doi: 10.3109/10837450.2016.1138130.
Mansur HS, Sadahira CM, Souza AN, Mansur AAP. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degrees and chemically crosslinked with glutaraldehyde. Mater Sci Eng C. 2008; 28(4):539–548. doi: 10.1016/j.msec.2007.10.088.
Pawar PG, Darekar A, Saudagar RB. Formulation, Development, and Evaluation of Febuxostat Loaded Microsponges. Int J Res Advent Technol. 2019;7(5):523–533. doi: 10.32622/ijrat.752019326.
Abd-Alhammid SN, Saleeh HH. Formulation and evaluation of flurbiprofen oral film. Iraqi J Pharm Sci. 2014; 23(1):53–59. doi: 10.31351/vol23iss1pp53-59.
Tamer MA, Abd-Al Hammid SN, Ahmed B. Formulation and in vitro evaluation of bromocriptine mesylate as fast dissolving oral film. Int J Appl Pharm. 2018;10(1):7-20. doi: 10.22159/ijap.2018v10i1.22615.
Aulton ME, Taylor K, eds. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, (5th ed.), Edinburgh: Churchill Livingstone /Elsevier; 2018. 36.
Nagy ZK, Balogh A, Vajna B, Farkas A, Patyi G, Kramarics Á, et al. Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci. 2012;101(1):322–332. doi: 10.1002/jps.22731.
Sundari PT, Lad K. Physico-chemical characterization and in vitro evaluation of biopharmaceutics classification system class II drug febuxostat. Asian J Pharm. 2016;10(2):S162-S169. doi: 10.22377/ajp.v10i2.640.
Kaur M, Mittal A, Gulati M, Sharma D, Kumar R. Formulation and in vitro evaluation of fast dissolving tablets of febuxostat using co-processed excipients. Recent Pat Drug Deliv Formul. 2020;14(1):48-62. doi: 10.2174/1872211314666191224121044.
Hadke A, Pethe A, Vaidya S, Dewani S. Formulation development and characterization of lyophilized febuxostat nanosuspension. Int J Appl Pharm. 2022;14(6):91-99. doi: 10.22159/ijap.2022v14i6.45614.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











