Role of Quercetin Supplementation on Iron Parameters in Blood Transfusion-Dependent Thalassemia Patients
DOI:
https://doi.org/10.54133/ajms.v7i1(Special).883Keywords:
Iron overload, Quercetin, ThalassemiaAbstract
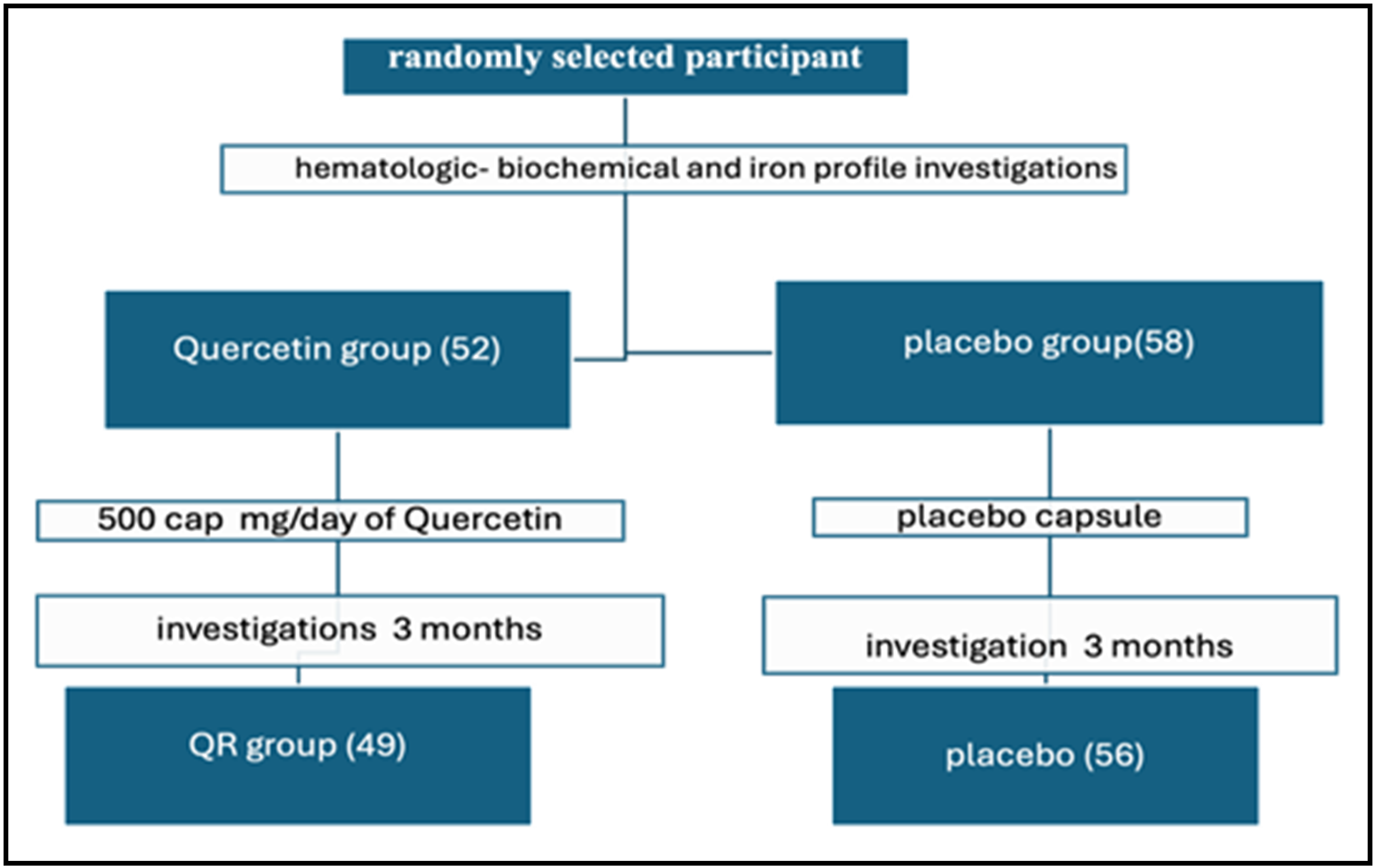
Background: Thalassemia is a group of inherited blood disorders that affect the production of hemoglobin, a protein in red blood cells that carries oxygen throughout the body. Iron overload is a condition in which the body absorbs and stores too much iron. In addition to repeated blood transfusions, increased gastrointestinal tract (GIT) iron absorption plays an important role in iron overload with thalassemia. Quercetin, a common flavonoid present in fruits and vegetables, exhibits diverse biological effects. Objective: To assess the effect of quercetin on iron overload parameters in blood transfusion-dependent thalassemia patients (TDT). Methods: A randomized, double-blind, placebo-placebo group-led study was conducted on 110 TDT patients, more than 12 years of age, who were supplemented with either quercetin or a placebo capsule daily (500 mg) for 3 months. A blood sample was obtained for laboratory parameters at baseline and at the end of 3 months. Results: At the baseline time of the study, the demographic features and iron overload parameters of patients and the placebo group were not statistically different, while after three months of supplementation, there was a significant decrease in levels of serum iron, UIBC, serum ferritin and ferritin saturation rate, and a significant increase in TIBC in the patients compared with the placebo group. Conclusions: The study shows the significant role of quercetin on iron overload parameters in blood transfusion-dependent thalassemia patients.
Downloads
References
Ali S, Mumtaz S, Shakir HA, Khan M, Tahir HM, Mumtaz S, et al. Current status of beta-thalassemia and its treatment strategies. Mol Genet Genomic Med. 2021;9(12):e1788. doi: 10.1002/mgg3.1788.
Putri IM, Gultom FP, Auerkari EI. Genetics and Epigenetics Aspects of Thalassemia. In4th International Conference on Life Sciences and Biotechnology (ICOLIB 2021) 2022 Dec 22 (pp. 288-296). Atlantis Press. doi: 10.2991/978-94-6463-062-6_28.
Thein SL. Molecular basis of β thalassemia and potential therapeutic targets. Blood Cells Mol Dis. 2018;70:54-65. doi: 10.1016/j.bcmd.2017.06.001.
Farmakis D, Porter J, Taher A, Cappellini MD, Angastiniotis M, Eleftheriou A. 2021 Thalassaemia international federation guidelines for the management of transfusion-dependent thalassemia. Hemasphere. 2022;6(8):e73. doi: 10.1097/hs9.0000000000000732.
Saha J, Panja A, Nayek K. The prevalence of HBB mutations among the transfusion-dependent and non transfusion-dependent Hb E/β-thalassemia children in a Tertiary Center of West Bengal, India. Hemoglobin. 2021;45(3):157-162. doi: 10.1080/03630269.2021.1933023.
Matzen AK, Woodland A, Beckett JR, Wood BJ. Oxidation state of iron and Fe-Mg partitioning between olivine and basaltic martian melts. Am Mineralogist. 2022;107(7):1442-1452. doi: 10.2138/am-2021-7682.
Man CD, Maideen SF, Rashid A. Knowledge, attitude and practice towards dietary iron among patients with thalassemia and their caregivers in Peninsular Malaysia. Med J Malaysia. 2019;74:365-371.
Sousa L, Oliveira MM, Pessôa MT, Barbosa LA. Iron overload: Effects on cellular biochemistry. Clin Chem Acta. 2020;504:180. doi: 10.1016/j.cca.2019.11.029.
Ansaf A, Faraj SA, Kazem S. The correlation between pituitary gonadotrophins, gonadal sex steroid hormone with ferritin level in pubertal females with thalassemia major at Wassit Province-Iraq 2020. Macedonian J Med Sci. 2021;9(B):806-810. doi: 10.3889/oamjms.2021.6611.
Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, El-Hack ME, Taha AE, et al. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020;9(3):374. doi: 10.3390%2Ffoods9030374.
Lesjak M, Balesaria S, Skinner V, Debnam ES, Srai SK. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur J Nutr. 2019;58:743-753. doi: 10.1007%2Fs00394-018-1680-7.
Pinto VM, Forni GL. Management of iron overload in beta-thalassemia patients: clinical practice update based on case series. Int J Mol Sci. 2020;21(22):8771. doi: 10.3390/ijms21228771.
Basu S, Rahaman M, Dolai TK, Shukla PC, Chakravorty N. Understanding the intricacies of iron overload associated with β-Thalassemia: A comprehensive review. Thalassemia Rep. 2023;13(3):179-194. doi: 10.3390/thalassrep13030017.
Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: A review. Molecules. 2022;27(9):2901. doi: 10.3390/molecules27092901.
Azeem M, Hanif M, Mahmood K, Ameer N, Chughtai FR, Abid U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polymer Bull. 2023;80(1):241-246. doi: 10.1007/s00289-022-04091-8.
Hezaveh ZS, Azarkeivan A, Janani L, Hosseini S, Shidfar F. The effect of quercetin in iron overload and inflammation in β-thalassemia major patients: A double-blind randomized clinical trial. Complement Ther Med. 2019;46:24-28. doi: 10.1016/j.ctim.2019.02.017.
Corrente GA, Malacaria L, Beneduci A, Furia E, Marino T, Mazzone G. Experimental and theoretical study on the coordination properties of quercetin towards aluminum (III), iron (III) and copper (II) in aqueous solution. J Mol Liquid. 2021;325:115171. doi: 10.1016/j.molliq.2020.115171.
Papan P, Kantapan J, Sangthong P, Meepowpan P, Dechsupa N. Iron (III)-quercetin complex: Synthesis, physicochemical characterization, and MRI cell tracking toward potential applications in regenerative medicine. Contrast Media Mol Imag. 2020;2020:1-22. doi: 10.1155/2020/8877862.
Lomozová Z, Catapano MC, Hrubša M, Karlíčková J, Macáková K, Kučera R, et al. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J Agricult Food Chem. 2021;69(21):5926-3. doi: 10.1021/acs.jafc.1c01729.
Xiao L, Luo G, Tang Y, Yao P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem Toxicol. 2018;114:190-203. doi: 10.1016/j.fct.2018.02.022.
Kejík Z, Kaplánek R, Masařík M, Babula P, Matkowski A, Filipenský P, et al. Iron complexes of flavonoids-antioxidant capacity and beyond. Int J Mol Sci. 2021;22(2):646. doi: 10.3390/ijms22020646
Ramavath HN, Konda V, Pullakhandam R. Quercetin inhibits hephaestin expression and iron transport in intestinal cells: Possible role of PI3K pathway. Nutrients. 2023;15(5):1205. doi: 10.3390/nu15051205.
Cotoraci C, Ciceu A, Sasu A, Hermenean A. Natural antioxidants in anemia treatment. Int J Mol Sci. 2021;22(4):1883. doi: 10.3390/ijms22041883.
Yin M, Liu Y, Chen Y. Iron metabolism: an emerging therapeutic target underlying the anti-cancer effect of quercetin. Free Radic Res. 2021;55(3):296-303. doi: 10.1080/10715762.2021.1898604.
Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, et al. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules. 2021;26(5):1315. doi: 10.3390/molecules26051315.
Zhao X, Wang J, Deng Y, Liao L, Zhou M, Peng C, et al. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021;35(9):4727-4747. doi: 10.1002/ptr.7104.
Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260-272. doi: 10.3324/haematol.2019.232124.
Al-Abedy NM, Salman ED, Faraj SA. Frequency of human hemochromatosis HFE gene mutations and serum hepcidin level in iron overload β-thalassaemia Iraqi patients. Public Health. 2019;22(10):S275. doi: 10.36295/ASRO.2019.220934.
Pang X, Zhong Z, Jiang F, Yang J, Nie H. Juglans regia L. extract promotes osteogenesis of human bone marrow mesenchymal stem cells through BMP2/Smad/Runx2 and Wnt/β-catenin pathways. J Orthop Surg Res. 2022;17(1):88. doi: 10.1186/s13018-022-02949-1.
Meng LQ, Yang FY, Wang MS, Shi BK, Chen DX, Chen D, et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate. 2018;78(11):790-800. doi: 10.1002/pros.23536.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











