Fabrication, Characterization and in vitro Evaluation of Prednisolone Sustained Release Multiparticulate System for Colonic Targeting
DOI:
https://doi.org/10.54133/ajms.v5i.253Keywords:
Colon target beads, Eudragit S-100, Guar gum, Inulin, Pectin, PrednisoloneAbstract
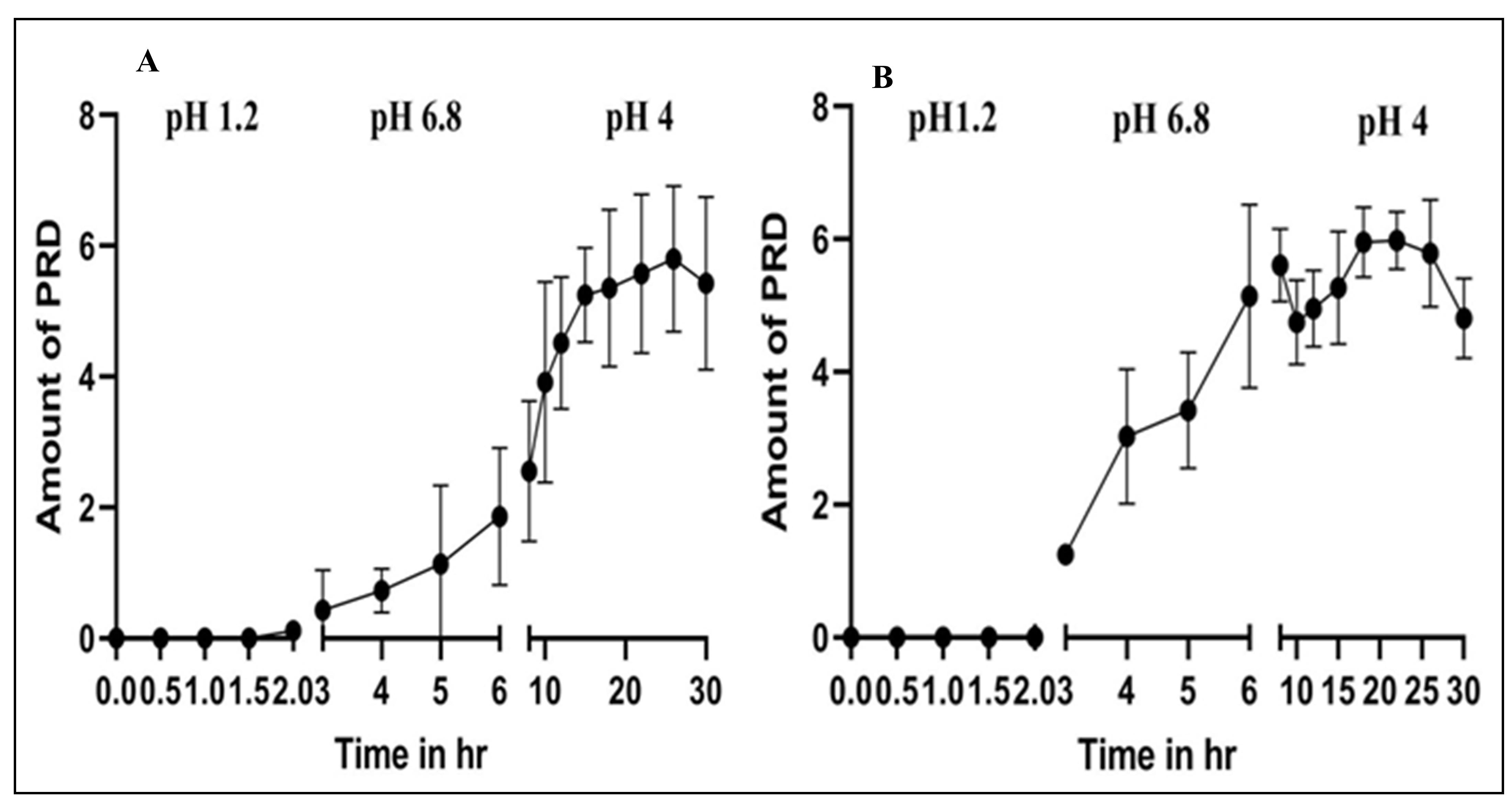
Background: Prednisolone (PRD) is orally prescribed for inflammatory bowel syndrome (IBS) as the upper GIT is the main site of absorption; therefore, long-term PRD dosing decreases therapeutic effectiveness through systemic side effects. Objective: This work focused on formulating sustained-release alginate beads as a multiparticulate system for colon targeting using prednisolone (PRD) to be filled in an HPMC capsule. Methods: PRD beads were prepared by the ionotropic gelation technique using sodium alginate as the primary polymer and inulin, guar gum, and pectin as secondary polymers. In addition to the impact of polymer type and quantity, other factors were investigated: The CaCl2 concentration and tween 80 addition Thirteen formulations were successfully prepared, and their properties, such as bead size, morphology, percentage of encapsulation efficiency, yield, DL, in vitro release study in GIT buffer media, IBS media, SEM, and FTIR, were assessed. Results: The study showed that the beads were close in size, and the size was not an obstacle for loading the beads in HPMC capsules. Further, yield%, EE%, and DL% increased according to the bead’s content increase. Conclusions: The optimum formula was F3 that coated HPMC capsules with Eudragit S-100, which gave sustained release profiles in GIT and IBS simulating media, and F13 that could last the release in different pH media, pH 1.2, 6.8, and 7.4.
Downloads
References
local disorders. Polysaccharide Carriers for Drug Delivery: Elsevier; 2019. p. 737-62.
Iswandana R, Putri KSS, Putri FA, Gunawan M, Larasati SA. Challenge and development strategy for colon-targeted drug delivery system. Pharm Sci Res. 2022;9(1):17-27.
Lozoya-Agullo I, Gonzalez-Alvarez I, Merino-Sanjuan M, Bermejo M, González-Álvarez M. Preclinical models for colonic absorption, application to controlled release formulation development. Eur J Pharm Biopharm. 2018;130:247-259. doi: 10.1016/j.ejpb.2018.07.008. DOI: https://doi.org/10.1016/j.ejpb.2018.07.008
Auriemma G, Cerciello A, Aquino RP, Del Gaudio P, Fusco BM, Russo P. Pectin and zinc alginate: The right inner/outer polymer combination for core-shell drug delivery systems. Pharmaceutics. 2020;12(2):87. doi: 10.3390/pharmaceutics12020087. DOI: https://doi.org/10.3390/pharmaceutics12020087
Mehdi-alamdarlou S, Mozafari N, Daneshamooz S, Ashrafi H. Preparation and in vitro evaluation of controlled release granules of mesalazine for colon targeted drug delivery system. Trends Pharm Sci. 2022;8(1):37-42. doi: 10.30476/tips.2021.92954.1116.
Sarangi MK, Rao MB, Parcha V. Smart polymers for colon targeted drug delivery systems: a review. Int J Polym Mater Polym Biomater. 2021;70(16):1130-66. doi: 10.1080/00914037.2020.1785455. DOI: https://doi.org/10.1080/00914037.2020.1785455
Lanjhiyana S. Polysaccharides based novel and controlled released multiparticulate systems for colon-specific delivery: Contemporary scenario and future prospects. Asian J Pharmaceutics. 2020;14(4). doi: 10.22377/ajp.v14i4.3815. DOI: https://doi.org/10.22377/ajp.v14i4.3815
Cerciello A, Auriemma G, Morello S, Aquino RP, Del Gaudio P, Russo P. Prednisolone delivery platforms: Capsules and beads combination for a right timing therapy. PLoS One. 2016;11(7):e0160266. doi: 10.1371/journal.pone.0160266. DOI: https://doi.org/10.1371/journal.pone.0160266
Gunter EA, Popeyko OV. Calcium pectinate gel beads obtained from callus cultures pectins as promising systems for colon-targeted drug delivery. Carbohydr Polym. 2016;147:490-499. doi: 10.1016/j.carbpol.2016.04.026. DOI: https://doi.org/10.1016/j.carbpol.2016.04.026
Bagyalakshmi J, Raj A, Ravi T. Formulation, physical characterization and in-vitro release studies of prednisolone alginate beads for colon targeting by ionotropic gelation. Pharmacie Globale. 2011;3:1-4.
Araujo V, Gamboa A, Caro N, Abugoch L, Gotteland M, Valenzuela F, et al. Release of prednisolone and inulin from a new calcium-alginate chitosan-coated matrix system for colonic delivery. J Pharm Sci. 2013;102(8):2748-2759. doi: 10.1002/jps.23656. DOI: https://doi.org/10.1002/jps.23656
Youcef Benzine. Enzymatically triggered polymeric drug delivery systems for colon targeting. Thesis: Human health and pathology. Université de Lille, 2019.
Patel N, Lalwani D, Gollmer S, Injeti E, Sari Y, Nesamony J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog Biomater. 2016;5:117-133. doi: 10.1007/s40204-016-0051-9. DOI: https://doi.org/10.1007/s40204-016-0051-9
Asnani GP, Bahekar J, Kokare CR. Development of novel pH–responsive dual cross-linked hydrogel beads based on Portulaca oleracea polysaccharide-alginate-borax for colon specific delivery of 5-fluorouracil. J Drug Deliv Sci Technol. 2018;48:200-208. doi: 10.1016/j.jddst.2018.09.023. DOI: https://doi.org/10.1016/j.jddst.2018.09.023
Vecino X, Devesa-Rey R, Cruz J, Moldes A. Study of the physical properties of calcium alginate hydrogel beads containing vineyard pruning waste for dye removal. Carbohydr Polym. 2015;115:129-138. doi: 10.1016/j.carbpol.2014.08.088. DOI: https://doi.org/10.1016/j.carbpol.2014.08.088
Ansari M, Sadarani B, Majumdar A. Colon targeted beads loaded with pterostilbene: Formulation, optimization, characterization and in vivo evaluation. Saudi Pharm J. 2019;27(1):71-81. doi: 10.1016/j.jsps.2018.07.021. DOI: https://doi.org/10.1016/j.jsps.2018.07.021
Helmy AM, Elsabahy M, Soliman GM, Mahmoud MA, Ibrahim EA. Development and in vivo evaluation of chitosan beads for the colonic delivery of azathioprine for treatment of inflammatory bowel disease. Eur J Pharm Sci. 2017;109:269-279. doi: 10.1016/j.ejps.2017.08.025. DOI: https://doi.org/10.1016/j.ejps.2017.08.025
Martínez-Terán M, Hoang-Thi T, Flament M. Multiparticulate dosage forms for pediatric use. Pediatr Ther. 2017;7:314. doi: 10.4172/2161-0665.1000314. DOI: https://doi.org/10.4172/2161-0665.1000314
Kaur N, Singh B, Sharma S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharma Innov J. 2018;7(7):142-148.
Sarangi M, Rao MB, Parcha V, Upadhyay A. Development and characterization of colon-targeting 5-fluorouracil multiparticulate beads. Indian J Pharm Sci. 2020;82(3):435-448. doi: 10.36468/pharmaceutical-sciences.666. DOI: https://doi.org/10.36468/pharmaceutical-sciences.666
Bale S, Khurana A, Reddy ASS, Singh M, Godugu C. Overview on therapeutic applications of microparticulate drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2016;33(4). doi: 10.1615/CritRevTherDrugCarrierSyst.2016015798. DOI: https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2016015798
Jung J, Arnold RD, Wicker L. Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloids Surf B Biointerfaces. 2013;104:116-121. doi: 10.1016/j.colsurfb.2012.11.042. DOI: https://doi.org/10.1016/j.colsurfb.2012.11.042
Jaiswal D, Bhattacharya A, Yadav IK, Singh HP, Chandra D, Jain D. Formulation and evaluation of oil entrapped floating alginate beads of ranitidine hydrochloride. Int J Pharm Pharm Sci. 2009;1(3):128-140.
Sarangi MK, Rao MB, Parcha V, Upadhyay A. Tailoring of colon targeting with sodium Alginate‐Assam bora rice starch based multi particulate system containing naproxen. Starch. 2020;72(7-8):1900307. doi: 10.1002/star.201900307. DOI: https://doi.org/10.1002/star.201900307
Atia A, Gomma AI, Fliss I, Beyssac E, Garrait G, Subirade M. Molecular and biopharmaceutical investigation of alginate–inulin synbiotic coencapsulation of probiotic to target the colon. J Microencapsul. 2017;34(2):171-184. doi: 10.1080/02652048.2017.1313330. DOI: https://doi.org/10.1080/02652048.2017.1313330
Das S. Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents. Int J Pharm. 2021;605:120814. doi: 10.1016/j.ijpharm.2021.120814. DOI: https://doi.org/10.1016/j.ijpharm.2021.120814
Osmałek T, Milanowski B, Froelich A, Szybowicz M, Białowąs W, Kapela M, et al. Design and characteristics of gellan gum beads for modified release of meloxicam. Drug Dev Indust Pharm. 2017;43(8):1314-1329. DOI: https://doi.org/10.1080/03639045.2017.1318896
Patole VC, Pandit AP. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: in vitro and in vivo characterization. J Pharm Invest. 2018;48(3):257-267. doi: 10.1007/s40005-017-0304-1. DOI: https://doi.org/10.1007/s40005-017-0304-1
Mar JM, da Silva LS, Rabello MDS, Biondo MM, Kinupp VF, Campelo PH, et al. Development of alginate/inulin carrier systems containing non-conventional Amazonian berry extracts. Food Res Int. 2021;139:109838. doi: 10.1016/j.foodres.2020.109838. DOI: https://doi.org/10.1016/j.foodres.2020.109838
Mohamed MBM, Qaddoori ZS, Hameed GS. Study the effect of 12-hydroxyoctadecanoic acid concentration on preparation and characterization of floating organogels using cinnarizin as modeling drug. Iraqi J Pharm Sci. 2022;31(2):169-176. doi: 10.31351/vol31iss2pp169-176. DOI: https://doi.org/10.31351/vol31iss2pp169-176
Mohammed MA, Kadhim KA, Jasim GA, Fawzi HA. Metformin compared to insulin for the management of gestational diabetic. Int J Res Pharm Sci. 2018;9(3):1063-1067. DOI: https://doi.org/10.26452/ijrps.v9i3.1630
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











