Preparation and Optimization of Olanzapine as Transdermal Nanoparticles Delivery System
DOI:
https://doi.org/10.54133/ajms.v6i2.786Keywords:
Nanoparticles, Nanoprecipitation, Olanzapine, Polymers, SolubilityAbstract
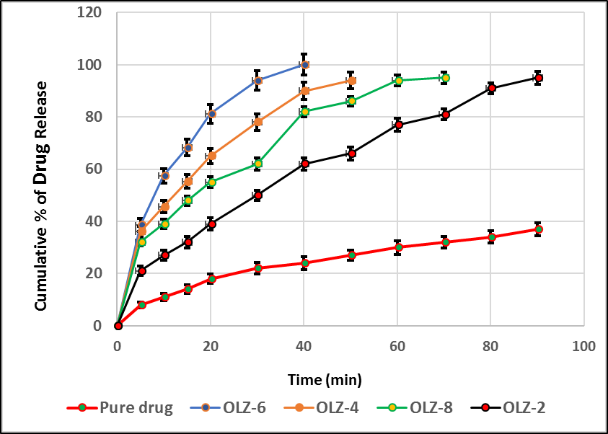
Background: The treatment of schizophrenia typically involves the use of olanzapine (OLZ), a typical antipsychotic drug that has poor oral bioavailability due to its low solubility and first-pass effect. Objective: To prepare and optimize OLZ as nanoparticles for transdermal delivery to avoid problems with oral administration. Methods: The nanoprecipitation technique was applied for the preparation of eight OLZ nanoparticles by using different polymers with various ratios. Nanoparticles were evaluated using different methods, including particle size, polydispersity index (PDI), entrapment efficiency (EE%), zeta potential and an in vitro release study. The morphology was evaluated by a field emission scanning electron microscope (FESEM) and an atomic force microscope (AFM). We also perform differential scanning calorimetry (DSC). Results: Characterization studies of OLZ nanoparticles showed that OLZ-6 was the best formula with a particle size of 115.76 nm, a PDI of 0.24, a high EE% of 78.4%, and a high zeta potential of -19.01 mV. The in vitro release of OLZ was higher than that of other formulations. FESEM reveals the spherical shape of the nanoparticles, and AFM screening confirms that the OLZ-6 size is comparable to what the Zeta sizer finds. The DSC results confirm the purity of OLZ and the compatibility between the drug and polymer. Conclusions: OLZ-6, as a transdermal delivery system, is a promising formula to overcome the problems associated with oral drug administration and could enhance its bioavailability.
Downloads
References
Abdulqader AA, Al-Khedairy EBH. Formulation and evaluation of fast dissolving tablets of taste-masked ondansetron hydrochloride by solid dispersion. Iraqi J Pharm Sci. 2017;26(1):50-60. doi: 10.31351/vol26iss1pp50-60.
Rubbens J, Mols R, Brouwers J, Augustijns P. Exploring gastric drug absorption in fasted and fed state rats. Int J Pharm. 2018;548(1):636–6641. doi: 10.1016/j.ijpharm.2018.07.017.
Adepu S, Ramakrishna S. Controlled drug delivery systems: Current status and future directions. Molecules. 2021;26(19):5905. doi: 10.3390/molecules26195905.
Szunerits S, Boukherroub R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front Bioeng Biotechnol. 2018;6(15):1-13. doi: 10.3389/fbioe.2018.00015.
Afzal O, Altamimi ASA, Nadeem MS, Alzarea SI, Almalki WH, Tariq A. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomaterials (Basel). 2022;12(24):4494. doi: 10.3390/nano12244494.
Alhagiesa AW, Ghareeb MM. Formulation and characterization of nimodipine nanoparticles for the Enhancement of solubility and dissolution rate. Iraqi J Pharm Sci. 2021;30(2):143-152. doi: 10.31351/vol30iss2pp143-152.
Muhesen RA, Rajab NA. Formulation and characterization of olmesartan medoxomil as a nanoparticle. Res J Pharm Technol. 2023;16(7):1-7. doi: 10.52711/0974-360X.2023.00547.
Zubiaur P, Soria-Chacartegui P, Villapalos-García G, Gordillo-Perdomo JJ, Abad-Santos F. The pharmacogenetics of treatment with olanzapine. Pharmacogenomics. 2021;22(14):939-958. doi: 10.2217/pgs-2021-005.
Alwan RM, Rajab NA. Nanosuspensions of selexipag: Formulation, characterization, and in vitro evaluation. Iraqi J Pharm Sci. 2021;30(1):144-153. doi: 10.31351/vol30iss1pp144-153.
Hamed HE, Hussein AA. Preparation, in vitro and ex-vivo evaluation of mirtazapine nanosuspension and nanoparticles incorporated in orodispersible tablets. Iraqi J Pharm Sci. 2020;29(1):62–75. doi: 10.31351/vol29iss1pp62-75.
Sreelola V, Sailaja AK, Pharmacy M. Preparation and characterisation of ibuprofen loaded polymeric nanoparticles by solvent evaporation technique. Int J Pharmacy Pharm Sci. 2014;6(8):416-421.
Mahmood HS, Ghareeb MM, Hamzah ZO. Formulation and in-vitro evaluation of flurbiprofen nanoparticles for transdermal delivery. J Complement Med Res. 2020;11(5):223.
Dalvi SV, Dave RN. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res. 2009;48(16):7581-7593. doi: 10.1021/ie900248f.
Rajab NA, Jassem NA. A design and in vitro evaluation of azilsartan medoxomil as a self-dispersible dry nanosuspension. Der Pharmacia Sinica. 2018;9(1):12-32.
Maher EM, Ali AM, Salem HF, Abdelrahman AA. In vitro/in vivo evaluation of an optimized fast dissolving oral film containing olanzapine co-amorphous dispersion with selected carboxylic acids. Drug Deliv. 2016;23(8):3088-3100. doi: 10.3109/10717544.2016.1153746.
Abbas IK, Abd AlHammid SN. Preparation and characterization of bilastine solid self-nanoemulsion using liquisolid technique. Al-Rafidain J Med Sci. 2023;5:78-85. doi: 10.54133/ajms.v5i.160.
Sehgal N, Gupta NV, Gowda DV. Fabrication and evaluation of solid dispersion containing Glibenclamide. Asian J Pharm Clin Res. 2018;11(8):158-161. doi: 10.22159/ajpcr. 2018.v11i8.26236.
Muneer R, Hashmet MR, Pourafshary P. DLVO modelling to predict critical salt concentration to initiate fines migration pre-and post-nano fluid treatment in sandstones. SPE J. 2022; 27:1915-1929. doi: 10.2118/209588-PA.
Cherukuri S, Batchu UR, Mandava K, Cherukuri V. Formulation and evaluation of transdermal drug delivery of topiramate. Int J Pharm Investig. 2017;7(1):10-17. doi: 10.4103/jphi.JPHI_35_16.
Ajit Shankarrao K, Dhairysheel Mahadeo G. Formulation and in-vitro evaluation of orally disintegrating tablets of olanzapine-2-Hydroxypropyl-β-Cyclodextrin inclusion complex. Iran J Pharm Res. 2010;9(4):335.
Hussien RM, Ghareeb MM. Formulation and characterization of isradipine nanoparticle for dissolution enhancement. Iraqi J Pharm Sci. 2021;30(1):218-225. doi: 10.31351/vol30iss1pp218-225.
Salatin S, Barar J, Barzegar-Jalali M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res Pharm Sci. 2017;12(1):1-14. doi: 10.4103/1735-5362.199041.
Liu D, Xu H, Tian B, Yuan K, Pan H, Ma S. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation-ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS Pharm SciTech. 2012;13(1):295-304. doi: 10.1208/s12249-011-9750-7.
Mishra R, Mir SR, Amin S. Polymeric nanoparticles for improved bioavailability of cilnidipine. Int J Pharm Pharm Sci. 2017;9(4):129-139. doi: 10.22159/ijpps.2017v9i4.15786.
Xu L, Chu Z, Zhang J. Steric effects in the deposition mode and drug-delivering efficiency of nanocapsule-based multilayer films. ACS Omega. 2022;7(34):30321-30332. doi: 10.1021/acsomega.2c03591.
Rudrangi SRS, Trivedi V, Mitchell JC. Preparation of olanzapine and methyl--cyclodextrin complexes using a single-step, organic solvent-free supercritical fluid process: An approach to enhance the solubility and dissolution properties. IJPR. 2015;494(1):408-416. doi: 10.1016/j.ijpharm.2015.08.062.
Ismail ST, Al-Kotaji MM, Khayrallah AA. Formulation and evaluation of nystatin microparticles as a sustained release system. Iraqi J Pharm Sci. 2015;24(2):1-10. doi: 10.31351/vol24iss2pp1-10.
Ali AH, Abd-Alhammid SN. Enhancement of solubility and Improvement of dissolution rate of atorvastatin calcium prepared as nanosuspension. Iraqi J PharmSci. 2019;28(2):46-57. doi: 10.31351/vol28iss2pp46-57.
Yang H, Teng F, Wang P, Tian B. Investigation of a nanosuspension stabilized by Soluplus® to improve bioavailability. Int J Pharm. 2014;30;477(1-2):88-95. doi: 10.1016/j.ijpharm.2014.10.025.
Tiwari M, Chawla G, Bansal AK. Quantification of olanzapine polymorphs using powder X-ray diffraction technique. J Pharm Biomed Anal. 2007;43(3):865-872. doi: 10.1016/j.jpba.2006.08.030.
Cho HW, Baek SH, Lee BJ, Jin HE. Orodispersible polymer films with the poorly water-soluble drug, olanzapine: hot-melt pneumatic extrusion for single-process 3D printing. Pharmaceutics. 2020;12(8):692. doi: 10.3390/pharmaceutics12080692.
Krishnamoorthy V, Suchandrasen, Verma Priya Ranjan Prasad VPR. Physicochemical characterization and in vitro dissolution behavior of olanzapine-mannitol solid dispersions. Braz J Pharm Sci. 2012;48(2):244-255. doi: 10.1590/S1984-82502012000200008.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











