Statistical Optimization and Characterization of Nimodipine Transferosomes
DOI:
https://doi.org/10.54133/ajms.v7i1(Special).1015الكلمات المفتاحية:
Box-Behenken design، Flux، Nimodipine، Transferosomesالملخص
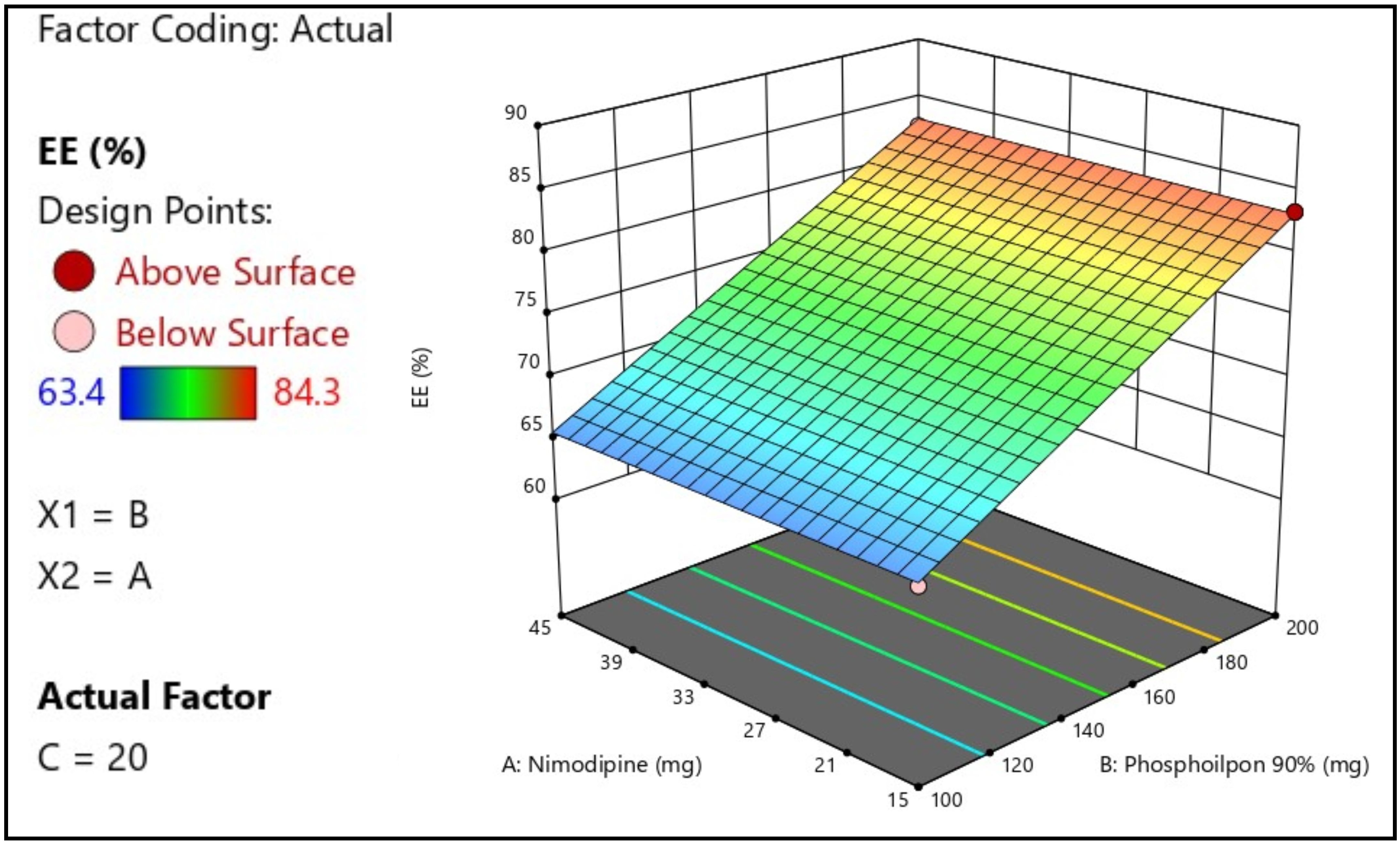
Background: Nimodipine is a vasodilator that is used for the prevention of cerebral vasospasm after subarachnoid hemorrhage. The oral and intravenous administration of the drug is associated with undesirable side effects. So, transdermal delivery using lipid-based nanovesicles, also known as transferosomes, can be thought of as an alternative. Objective: To optimize the formulation of transferosomes using the statistical design of experiments, with the aim of obtaining the most suitable transferosomes for the transdermal delivery of nimodipine. Methods: In the Box-Behenken statistical design, the independent variables were the quantities of nimodipine, phospholipon 90%, and sodium deoxycholate, while the dependent variables were the vesicle size, entrapment efficiency for nimodipine and its flux through the rat's skin. The optimized formulation was characterized through transmission electron microscopy and the deformability index. Results: The optimized formulation of transferosomes suggested by the software consisted of 30 mg nimodipine, 150 mg phospholipon 90% and 15 mg sodium deoxycholate. The resulted values were 248 nm for vesicles size, 81% for entrapment, and 476 μg/cm2/h. Under transmission electron microscopy, transferosomes appeared as vesicles, with a 0.98 deformability index for the optimized formula. Conclusions: Nimodipine can be formulated as transferosomes and efficiently applied for transdermal delivery.
التنزيلات
المراجع
Prakash S. Nano-based drug delivery system for therapeutics: a comprehensive review. Biomed Phys Engineer Express. 2023;9(5):1976-2157. doi: 10.1088/2057-1976/acedb2.
Sadeq ZA, Rajab NA, Abd Alhammid SN, Zaki H. Preparation, in-vitro evaluation, mechanical characterization, and release of nebivelol hydrochloride as A transdermal film using combined eudragite-polyvinyl alcohol as adhesive film forming polymer. J Pharm Sci Res. 2019;11(3):1052-1055.
Mirtaleb MS, Shahraky MK, Ekrami E, Mirtaleb A. Advances in biological nanophospholipid vesicles for transdermal delivery: A review on applications. J Drug Del Sci Tech. 2021; 61:102331. doi: 10.1016/j.jddst.2021.102331.
Jain S, Patel N, Shah MK, Khatri P, Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2017;106(2):423-445. doi: 10.1016/j.xphs.2016.10.001.
Thomas LM, Khasraghi AH. Nanotechnology-based topical drug delivery systems for management of dandruff and seborrheic dermatitis: An overview. Iraqi J Pharm Sci. 2020;29(1):12-32. doi: 10.31351/vol29iss1pp12-32.
Garg G, Jain K. Dermal and transdermal drug delivery through vesicles and particles: Preparation and applications. Adv Pharm Bull. 2022;12(1):45-57. doi: 10.34172/apb.2022.006.
Joshi A, Kulkarni R, Chaudhari R. In-vitro and ex-vivo evaluation of raloxifene hydrochloride delivery using nanotransfersome based formulations. J Drug Del Sci and Tech. 2018;45:151-158. doi: 10.1016/j.jddst.2018.02.006.
Mohammed BS, Al Gawhari FJ. Transethosomes a novel transdermal drug delivery system for antifungal drugs. Int J Drug Del Tech. 2021;11(1):238-243. doi: 10.25258/ijddt.11.1.45.
Salih OS, Al-Akkam EJ. Preparation, in vitro, and ex vivo evaluation of ondansetron loaded invasomes for transdermal delivery. Iraqi J Pharm Sci. 2023;32(3):71-84. doi: 10.31351/vol32iss3pp71-84.
Fareed NY, Kassab HJ. Diacerein loaded novasome for transdermal delivery: preparation, in-vitro characterization and factors affecting formulation. Iraqi J Pharm Sci. 2023;32(Suppl.). doi: 10.31351/vol32issSuppl.pp214-224.
Alkwak RS, Rajab NA. Lornoxicam-loaded cubosomes: preparation and in vitro characterization. Iraqi J Pharm Sci. 2021;31(1):144-153. doi: 10.31351/vol31iss1pp144-153.
Zylberberg C, Sandro Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Del. 2016;23(9):3319-3329. doi: 10.1080/10717544.2016.1177136.
Singh D, Pradhan M, Nag M, Singh MR. Vesicular system: versatile carrier for transdermal delivery of bioactives. Artif Cell Nanomed Biotechnol. 2015;43:282-290. doi: 10.3109/21691401.2014.883401.
Fernández-García R, Lalatsa A, Statts L, Bolás-Fernández F, Ballesteros MP, Serrano DR. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int J Pharm 2020;573:118817. doi: 10.1016/j.ijpharm.2019.118817.
Shakthi Apsara Thejani Opatha, Varin Titapiwatanakun and Romchat Chutoprapat. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12:855. doi: 10.3390/pharmaceutics12090855.
Rai S., Pandey V, Rai G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev Exp. 2017;8:1325708. doi: 10.1080/20022727.2017.1325708.
Mahdi MB, Aliasghar A, Kadhim B. Sixty-four multi-slice cerebral CT angiographic findings in early non-traumatic subarachnoid hemorrhage. J Fac Med Baghdad. 2014;56(2):151-156.
Winkler SR. Stroke, In: Chisholm-Burns MA, Schwinghammer TL, Wells BG, Malone PM, Kolesar JM, DiPiro JT, (Eds.), Pharmacotherapy Principles & Practice, (11th ed.), New York: McGraw-Hill Education; 2016. pp. 193-205.
Rass V, Kindl P, Lindner A, Kofer M, Altmann K, Putnina L, et al. Blood pressure changes in association with nimodipine therapy in patients with spontaneous subarachnoid hemorrhage Neurocrit Care. 2023;39:104-115. doi: 10.1007/s12028-023-01760-y.
Mahmoud SH, Ji1 X, Isse FA. Nimodipine pharmacokinetic variability in various patient populations. Drugs R D. 2020;20:307–318. doi: 10.1007/s40268-020-00322-3.
Ahlam Zaid Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438-470. doi: 10.3390/pharmaceutics/7040438.
Varia U, Joshi D, Jadeja M, Katariya H, Detholia K, Soni V. Development and evaluation of ultradeformable vesicles loaded transdermal film of boswellic. Future J Pharm Sci. 2022;8:39. doi: 10.1186/s43094-022-00428-2.
Wu PS, Li YS, Kuo YC, Tsai SJ, Lin CC. Preparation and evaluation of novel transfersomes combined with the natural antioxidant resveratrol. Molecules. 2019;24:600;1-12. doi: 10.3390/molecules24030600.
Omar MM, Hasan OA, El Sisi AM. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: a promising approach for enhancement of skin permeation. Int J Nanomedicine. 2019;14:1551-1562. doi: 10.2147/IJN.S201356.
Qushawy M, Nasr A, Abd-Alhaseeb M, Swidan S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics. 2018;10:26. doi: 10.3390/pharmaceutics10010026.
Krishnaiah YS, Bhaskar P, Satyanarayana V. In vitro percutaneous permeability enhancement of nimodipine by limonene across the excised rat abdominal skin. Pharmazie. 2004;59(12):942-947. PMID: 15638083.
Moffat AC, Osselton MD, Widdop B (Eds.), Clarke’s Analysis of Drugs and Poisons, (4th ed.), London: The Pharmaceutical Press; 2011.
Fahmy AM, Hassan M, El-Setouhy DA, Tayel SA, Al-Mahallawi AM. Statistical optimization of hyaluronic acid enriched ultradeformable elastosomes for ocular delivery of voriconazole via Box-Behnken design: in vitro characterization and in vivo evaluation. Drug Del. 2021;28(1):77-86. doi: 10.1080/10717544.2020.1858997.
Almehmady AM, Elsisi AM. Development, optimization, and evaluation of tamsulosin nanotransfersomes to enhance its permeation and bioavailability. J Drug Del Sci Tech. 2020;57:101667. doi: 10.1016/j.jddst.2020.101667.
Raza K, Singh B, Mahajan A, Negi P, Bhatia A, Katare OP. Design and evaluation of flexible membrane vesicles (FMVs) for enhanced topical delivery of capsaicin. J Drug Target. 2011;19(4):293-302. doi: 10.3109/1061186X.2010.499464.
Fukuda IM, Pinto CF, Moreira CD, Saviano AM, Lourenço FR. Design of Experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci. 2018;54(Special):e01006. doi: 10.1590/s2175-97902018000001006.
Singh S, Vardhan H, Kotla NG, Maddiboyina B, Sharma D, Webster TJ. The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic. Int J Nanomedicine. 2016;11:1475-82. doi: 10.2147/IJN.S100253.
Aghdam MH, Ghanbarzadeh, Javadzadeh Y, Hamishehkar H. Aggregated transfersomal dry powder inhalation of itraconazole for pulmonary drug delivery. Adv Pharm Bull. 2016;6:57-64. doi: 10.15171/apb.2016.009.
Singh S, Vardhan H, Kotla NG, Maddiboyina B, Sharma D, Webster TJ. The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic. Int J Nanomedicine. 2016;11:1475-1482. doi: 10.2147/IJN.S100253.
35 Ahmed TA, Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J Liposome Res. 2015;25:1-10. doi: 10.3109/08982104.2014.950276.
Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. Effect of edge activator on characteristic and in vitro skin permeation of meloxicam loaded in elastic liposomes. Adv Mater Res. 2011;194-196:537–540. doi: 10.4028/AMR.194-196.537.
Duangjit S, Obata Y, Sano H, Onuki Y, Opanasopit P, Ngawhirunpat T, et al. Comparative study of novel ultradeformable liposomes: menthosomes, transfersomes and liposomes for enhancing skin permeation of meloxicam. Biol Pharm Bull. 2014;37(2):239-47. doi: 10.1248/bpb.b13-00576.
Rajab NA, Jawad MS. Preparation and evaluation of rizatriptan benzoate loaded nanostructured lipid carrier using different surfactant/co-surfactant Systems. Int J Drug Del Tech. 2023;13(1):120-126.
Bragagni M, Mennini N, Maestrelli F, Cirri M, Mura P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Del. 2012;(19):354-361. doi: 10.3109/10717544.2012.724472.
Kamani P, Parikh K, Kapadia R, Sawant K. Phospholipid based ultra-deformable nanovesicular gel for transcutaneous application: QbD based optimization, characterization and pharmacodynamic profiling. J Drug Del Sci Tech. 2019;(51):152-163.
Ben Mustapha RB, Lafforgue C, Fenina N, Marty JP. Influence of drug concentration on the diffusion parameters of caffeine. Indian J Pharmacol. 2011; (43):157-162. doi: 10.4103/0253-7613.77351.
Parhi R, Swain S. Transdermal evaporation drug delivery system: Concept to commercial products. Adv Pharm Bull. 2018;(8):535-550. doi: 10.15171/apb.2018.063.
التنزيلات
منشور
كيفية الاقتباس
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2024 Al-Rafidain Journal of Medical Sciences

هذا العمل مرخص بموجب Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).










