Formulation, Development and Evaluation of Sildenafil Citrate Oral Jelly
DOI:
https://doi.org/10.54133/ajms.v5i.178الكلمات المفتاحية:
Sildenafil، Jelly، Spreadability، Extrudability، pH، Stabilityالملخص
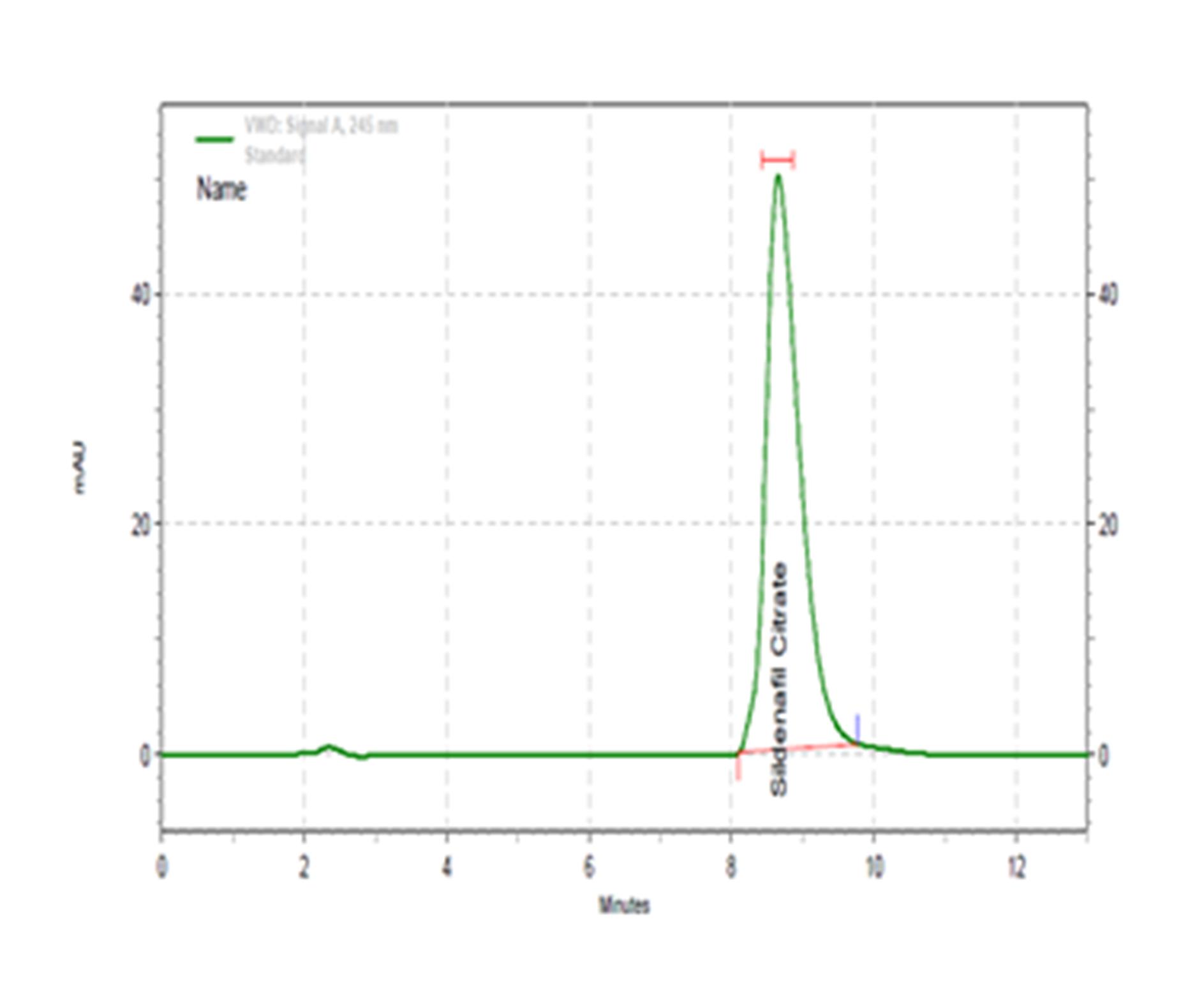
Background: The site of drug administration, such as oral or intravenous, frequently categorizes the route of administration. Drug delivery through oral jelly reduces product costs and enhances product stability and appearance. Oral jelly is used as a drug carrier for different diseases like erectile dysfunction, arthritis, hypertension, and sore throats. Medicated jelly is easy to administer at any time and place without water. Aim: To formulate, develop, and evaluate sildenafil citrate oral jelly. Methods: Oral jelly formulations (F1–F5) with different amounts of excipients and sildenafil citrate were made and tested for things like pH, appearance, viscosity, and drug release in a test tube. The data were analyzed by SPSS version 25 software. Results: The pre-formulation FTIR study revealed that there was no significant interaction between the drug and the excipients used. The pH of all of the formulations was within the desirable range (6.3-6.8), indicating their suitability for product stability and patient acceptability. The stability study indicated that during one-month storage at 25⁰ C and at 40⁰ C/ 75% RH, no significant changes in the properties of the product were found. The viscosity of the formulations increased with increasing sodium CMC concentrations. The HPLC-based in vitro drug release study indicated that the best drug release was achieved in the F5 formulation. Conclusion: It was concluded that F5 is the formulation of choice, satisfying the ideal characteristics of an oral jelly formulation with an improvement in the drug bioavailability over the existing marketed oral formulations of Sildenafil.
التنزيلات
المراجع
Ummadi S, Shravani B, Rao NR, Reddy MS, Sanjeev B. Overview on controlled release dosage form. Int J Pharm Sci. 2013;3(4):258-269.
Mathias NR, Hussain MA. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J Pharm Sci. 2010;99 (1):1-20. doi: 10.1002/jps.21793. DOI: https://doi.org/10.1002/jps.21793
Jain DK, Darwhekar GN, GuptaV. Formulation and evaluation of oral soft jelly containing metformin hydrochloride and glimepiride. Indo Am J Pharm Sci. 2016;3(10):1276-1282. doi: 10.5281/zenodo.167095.
Sarojini S, Anusha K, Maneesha C, Mufaquam MA, Deepika B, Krishna Y, et al. Oral Medicated Jellies–a Review. World J Pharm Res. 2018;7(6):352-365. doi: 10.20959/wjpr20186-11502.
Alberti I, Grenier A, Kraus H, Carrara DN. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin Drug Deliv. 2005;2(5):935-950. doi: 10.1517/17425247.2.5.935. DOI: https://doi.org/10.1517/17425247.2.5.935
Prabhu C. Formulation development and evaluation of tadalafil oral jelly comparative with marketed product. 2014; [Thesis] https://core.ac.uk/download/pdf/235653475.pdf [Accessed on 8th August 2022]
Raja Manali M, Dhiren P. Oral medicated jelly: a recent advancement in formulation. Int J Pharm Sci. 2016;7(2):13-20.
Yadav C, Tangri S, Yadav R. A review recent advancement in formulation of oral medicated jelly. World J Pharmacy Pharm Sci. 2018;7(7):417-426. doi: 10.20959/wjpps20187-11945.
Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5S-12S. doi: 10.1046/j.0306-5251.2001.00027.x. DOI: https://doi.org/10.1046/j.0306-5251.2001.00027.x
Mohamed MI. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004;6(3):e26. doi: 10.1208/aapsj060326. DOI: https://doi.org/10.1208/aapsj060326
Badmaev V, Majeed M, Prakash L. Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation. J Nutr Biochem. 2000;11(2):109-113. doi: 10.1016/s0955-2863(99)00074-1. DOI: https://doi.org/10.1016/S0955-2863(99)00074-1
Ibrahim KA, Nawaz A, Mumtaz S, Iqbal FM, Khan A, El-Salam A, et al. Formulation, evaluation and release rate characteristics of medicated jelly of vitamin C. Pak J Pharm Sci. 2017;30(2(Suppl.)):579-583.
Prakash K, Satyanarayana VM, Nagiat HT, Fathi AH, Shanta AK, Prameela AR. Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum, and gellan gum. Asian J Pharm. 2014;8:241-249. doi: 10.4103/0973-8398.143937. DOI: https://doi.org/10.4103/0973-8398.143937
Hosseini E, Mozafari HR, Hojjatoleslamy M, Rousta E. Influence of temperature, pH and salts on rheological properties of bitter almond gum. Food Sci Technol. 2017;37:437-443. doi: 10.1590/1678-457X.18116. DOI: https://doi.org/10.1590/1678-457x.18116
Binder L, Mazál J, Petz R, Klang V, Valenta C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res Technol. 2019;25(5):725-734. doi: 10.1111/srt.12709. DOI: https://doi.org/10.1111/srt.12709
التنزيلات
منشور
كيفية الاقتباس
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2023 Al-Rafidain Journal of Medical Sciences

هذا العمل مرخص بموجب Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).










