Preparation and Evaluation of Solid Dispersion-Based Bilastine Effervescent Granules
DOI:
https://doi.org/10.54133/ajms.v6i2.806الكلمات المفتاحية:
Bilastine، Effervescent granules solid dispersion، Kneading technique، PVP K30، PLX188الملخص
الخلاصة: البلاستين دواء من الجيل الثاني من مضادات الهستامين لعلاج الالتهاب التحسسي للملتحمة الانفية،يصنف البلاستين لقلة ذوبانيته في الماء كدواء من الصنف الثاني. اثبتت تقنية المتشتت الصلب فعاليتها في تحسين ذوبان وسرعة تحرر العديد من الادوية غير الذائبة.
الهدف: تحسين ذوبانية وتحرر البلاستين عن طريقه تحضيره بصيغة المتشتت الصلب لغرض تحويله الى حبيبات فوارة.
الطرق: تم استخدام طريقة العجن لتحضير المتشتت الصلب للبلاستين باستخدام البولوكزامر188 و فينيل بيرلوريدون ك 30 وبالنسب 1:5و1:10و1:15 من الدواء والحامل المائي. قيمت جميع الصيغ من ناحية النسبة الانتاجية ومحتوى الدواء وقابلية الذوبان المائية. ثم خضعت الصيغ ذات التحسن العالي للذوبانية لاختبار سرعة مدى تحرر الدواء. تم تحليل الصيغة المثالية للمتشتت الصلب حراريا بوساطة المسح التفاضلي الكالوري، حيود الاشعة السينية، و باستخدام التحليل الطيفي للاشعة تحت الحمراء تم دراسة التفاعل بين الدواء والناقل المائي. حضرت صيغة المتشتت الصلب المثالية كحبيبات فوارة باستخدام التحبيب الرطب وخضوعها للتقييم ايضا.
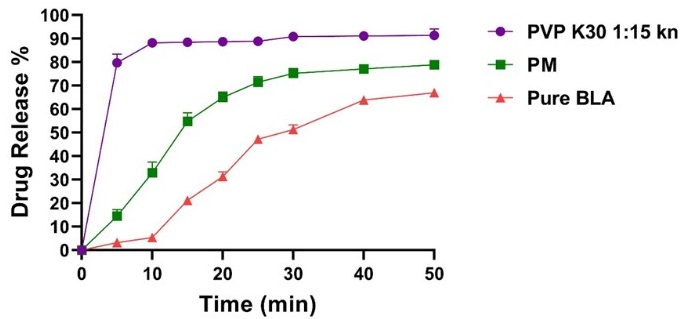
النتائج:اظهرت صيغة المتشتت الصلب للبلاستين المحضرة باستخدام البولي فينيل بيرلوريدون ك 30 بنسبة 1:15 من الدواء والحامل المائي اعلى تحسين للذوبانية المائية واسرع تحرر للدواء,حيث حررت اكثر من 88.43% من الدواء في اول 15 دقيقة في الوسط الفوسفاتي ذو الرقم الهيدروجيني 6.8. كما أظهرت الحبيبات الفوارة المحضرة من صيغة المتشتت الصلب المثالية خصائص تدفق ممتازة، ووقت تفكك قدره 87 ثانية، ودرجة حموضة مقبولة تبلغ 5.9 و9.7 ملجم من الدواء المذاب في أول 5 دقائق.
الاستنتاج: تم تحسين ذوبانية دواء البلاستين بواسطة تقنية التشتت الصلب بما ساعد في تحضيره كحبيبات فوارة
التنزيلات
المراجع
Ruba Malkawi WIM, Mahmoud Y, Tawalbeh J. Current trends on solid dispersions: past, present, and future. Adv Pharmacol Pharm Sci. 2022;2022:5916013. doi: 10.1155/2022/5916013.
De Mohac LM, Raimi-Abraham B, Caruana R, Gaetano G, Licciardi M. Multicomponent solid dispersion a new generation of solid dispersion produced by spray-drying. J Drug Deliv Sci Technol. 2020;57:101750. doi: 10.1016/j.jddst.2020.101750.
Nikghalb LA, Singh G, Singh G, Kahkeshan KF. Solid dispersion: methods and polymers to increase the solubility of poorly soluble drugs. J Appl Pharm Sci. 2012;2(10):170-175. doi: 10.7324/JAPS.2012.21031.
Shankarguru P, Ramya Ddevi D, Hari Bn V. Effect of water content in kneading method of solid dispersion technique for solubility enhancement. Int J App Pharm. 2017;9. doi: 10.22159/ijap.2017v9i5.17765.
Hatem AQ, Ali WK. Preparation and characterization of carvedilol solid dispersion by kneading method. Al Mustansiriyah J Pharm Sci. 2023;23:367-377. doi: 10.32947/ajps.v23i4.1092.
Leceta A, García A, Sologuren A, Campo C. Bilastine 10 and 20 mg in paediatric and adult patients: an updated practical approach to treatment decisions. Drugs Context. 2021;10:1-15. doi: 10.7573/dic.2021-5-1.
Nechipadappu SK, Swain D. Combined synthetic and solubility aspects of orotate salt of bilastine. J Mol Struct. 2023;1271:1-4. doi; 10.1016/j.molstruc.2022.134148.
Ghareeb MM, Abdulrasool AA, Hussein AA, Noordin MI. Kneading technique for preparation of binary solid dispersion of meloxicam with poloxamer 188. AAPS PharmSciTech. 2009;10(4):1206-1215. doi: 10.1208/s12249-009-9316-0.
Jejurkar L, Tapar KK. Preparation and characterization of mesalamine solid dispersions by kneading method. IJPSR. 2011;2(10):2623-2628. doi: 10.13040/IJPSR.0975-8232.2(10).2623-28.
Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS PharmSciTech. 2017;7(3):68. doi; 10.1208%2Fpt070368.
Al-Khedairy EB, Hussein LS. Solubility and dissolution enhancement of ebastine by surface solid dispersion technique. Iraqi J Pharm Sci. 2021;30:122-132. doi: 10.31351/vol30iss1pp122-132.
Eltayeeb A, Al-Khedairy EB. Preparation and evaluation of aceclofenac solid dispersion by fusion technique and effervescent assisted fusion technique: comparative study. Res J Pharm Technol. 2023:5358-5365. doi: 10.52711/0974-360X.2023.00868.
Rekha K, Aruna RM, Rekha MM, Karthik S. Formulation and development of bilastine tablets 20 mg. World J Pharm Res. 2019;8:2197-224.
Ahmed KK, Kassab HJ, Al Ramahi IJ, Alwan ZS. Taste masking of steroids for oral formulations. Turk J Pharm Sci. 2024;20(6):352-360. doi: 10.4274/tjps.galenos.2023.24968.
Salim FF, Rajab NA. Formulation and characterization of piroxicam as self-nano emulsifying drug delivery system. Iraqi J Pharm Sci. 2020;29:174-183. doi: 10.31351/vol29iss1pp174-183.
Hussein ZK, Al-Kinani KK. Formulation and evaluation of the risperidone solid dispersion using different carriers. J Res Med Dent Sci. 2023;7:26-34.
Divya K, Vamshi G, Vijaykumar T, Rani MS, Kishore B. Review on introduction to effervescent tablets and granules. Kenkyu J Pharmacol. 2020;6:1-9.
Al-Mousawy J, Al-Hussainy Z, Alaayedi M. Formulation and evaluation of effervescent granules of ibuprofen. Int J Appl Pharm. 2019:66-69. doi: 10.22159/ijap.2019v11i6.34912.
Alwan ZS, Ibrahim MA. Study the effect of disintegrant types on preparation and in vitro evaluation of salbutamol sulfate effervescent granules. Kerbala J Pharm Sci. 2021;1(19):1-9.
Sangram Biranje AM, Trusha P, Shangrapawar AB. A review on formulation and evaluation of effervescent tablet. IJPPR. 2021;21:477-486.
Diyya ASM, Thomas NV. Formulation and evaluation of metronidazole effervescent granules. Int J Pharm Sci Res. 2018;9:2525-2529. doi: 10.13040/IJPSR.0975-8232.9(6).2525-29.
Basavaraj VB, Saritha N, Bharath S, Madhavan V. Vigna mungo mucilage- a natural polymer in the design of matrix based SR tablet of aceclofenac. Int J Pharm Sci Rev Res. 2013;21(2).
Sonawane A, Sudhir S, Pathak RCP. Effect of drying on physical and flow properties of banana powder. Carpathian J Food Sci Technol. 2021:174-185. doi: 10.34302/crpjfst/2021.13.3.14.
Ayan Pani SP, Kanthal LK, Bera M, Jana P, Samanta S, Izaz Ahmed Khan IA, et al. A comparative assessment of granulation methods containing effervescent granules of vitamin C. J Res Pharm Sci. 2022;8(2022):32-39.
Aslani A, Fattahi F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. Adv Pharm Bull. 2013;3(1):217-225. doi: 10.5681%2Fapb.2013.036.
Hussain HAM, Al-Khedairy EB. Preparation and in vitro evaluation of cyclodextrin based effervescent and dispersible granules of carbamazepine. Int J Appl Pharm. 2018;10(6). doi: 10.22159/ijap.2018v10i6.28276.
Abbas IK, Al Hammid SNA. Preparation and characterization of bilastine solid self- nanoemulsion using liquisolid technique. Al-Rafidain J Med Sci. 2023;5:78-85. doi: 10.54133/ajms.v5i.160.
Ladhake V, Kadu S, Tayade V, Gawand S, Malviya V. Preparation and evaluation of oral fast dissolving films of bilastine using pullulan. IJSDR. 2020;5:282-287.
El Maghraby GM, Elsergany RN. Fast disintegrating tablets of nisoldipine for intra-oral administration. Pharm Devel Technol. 2014;19(6):641-650. doi: 10.3109/10837450.2013.813543.
Ardiansyah A, Nasrul E, Rivai H, Sahlan Ben E, Zaini E. Physicochemical characterization of amorphous solid dispersion of ketoprofen–polyvinylpyrrolidone k-30. Int J Pharmacy Pharm Sci. 2014;7:209-212.
Liu P, Zhou JY, Chang JH, Liu XG, Xue HF, Wang RX, et al. Soluplus-mediated diosgenin amorphous solid dispersion with high solubility and high stability: development, characterization and oral bioavailability. Drug Des Dev Ther. 2020;14:2959-2975. doi: 10.2147%2FDDDT.S253405.
harma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49-56. PMID: 21589768.
Sharannavar BR, Gadad AP. Physicochemical characterization and dissolution study of spray dried amorphous lovastatin with Polyvinylpyrrolidone K30. Pharma Innov J. 2018;7(3):498-502.
Liw JJ, Teoh XY, Teoh AXY, Chan SY. The effect of carrier-drug ratios on dissolution performances of poorly soluble drug in crystalline solid dispersion system. J Pharm Sci. 2022;111(1):95-101. doi: 10.1016/j.xphs.2021.06.026.
Jassim BM, Al-Khedairy EB. Enhancement of silymarin solubility and dissolution by nicotinamide-based solid dispersion employing the kneading method. JPTCP. 2023;30(13). doi: 10.47750/jptcp.2023.30.13.028.
Rusdin A, Mohd Gazzali A, Ain Thomas N, Megantara S, Aulifa DL, Budiman A, et al. Advancing drug delivery paradigms: Polyvinyl pyrolidone (PVP)-based amorphous solid dispersion for enhanced physicochemical properties and therapeutic efficacy. Polymers (Basel). 2024;16(2):286. doi: 10.3390/polym16020286.
Homayouni A, Sadeghi F, Nokhodchi A, Varshosaz J, Garekani HA. Preparation and characterization of celecoxib solid dispersions; comparison of poloxamer-188 and PVP-K30 as carriers. Iran J Basic Med Sci. 2014;17(5):322-331. PMID: 24967060.
Noolkar SB, Jadhav NR, Bhende SA, Killedar SG. Solid-state characterization and dissolution properties of meloxicam-moringa coagulant-PVP ternary solid dispersions. AAPS PharmSciTech. 2013;14(2):569-577. doi: 10.1208/s12249-013-9941-5.
Bashar KKG, Al-Khedairy EB. Solubility and dissolution enhancement of atorvastatin calcium using phospholipid solid dispersion technique. Iraqi J Pharm Sci. 2023;32:244-253. doi: 10.31351/vol32issSuppl.pp244-253.
Del Río Pericacho JL, Lopez RP, Arredondo Martinez YE. Crystalline forms of bilastine and preparation methods thereof. European Patent Application (EP 3 327 012 A1). 2018.
Iyer R, Petrovska Jovanovska V, Berginc K, Jaklič M, Fabiani F, Harlacher C, et al. Amorphous solid dispersions (ASDs): The influence of material properties, manufacturing processes and analytical technologies in drug product development. Pharmaceutics. 2021;13(10):1682. doi: 10.3390/pharmaceutics13101682.
Rosiak N, Wdowiak K, Tykarska E, Cielecka-Piontek J. Amorphous solid dispersion of hesperidin with polymer excipients for enhanced apparent solubility as a more effective approach to the treatment of civilization diseases. Int J Mol Sci. 2022;23(23):15198. doi: 10.3390/ijms232315198.
Huynh DTM, Hai HT, Hau NM, Lan HK, Vinh TP, Tran V, et al. Preparations and characterizations of effervescent granules containing azithromycin solid dispersion for children and elder: Solubility enhancement, taste-masking, and digestive acidic protection. Heliyon. 2023;9(6):e16592. doi: 10.1016/j.heliyon.2023.e16592.
Attebäck M, Hedin B, Mattsson S. Formulation, optimization of extemporaneous oral liquids containing naloxone and propranolol for pediatric use. Scientia Pharmaceutica. 2022;90(1). doi: 10.3390/scipharm90010015.
التنزيلات
منشور
كيفية الاقتباس
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2024 Al-Rafidain Journal of Medical Sciences

هذا العمل مرخص بموجب Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).










