Formulation and Evaluation of Immediate-Release Oral Tablets Containing Magnesium Aluminum Silicate-Loaded Simvastatin
DOI:
https://doi.org/10.54133/ajms.v6i2.745Keywords:
Adsorption technique, Immediate-release tablets, Magnesium Aluminum silicate, Simvastatin, Soluplus®Abstract
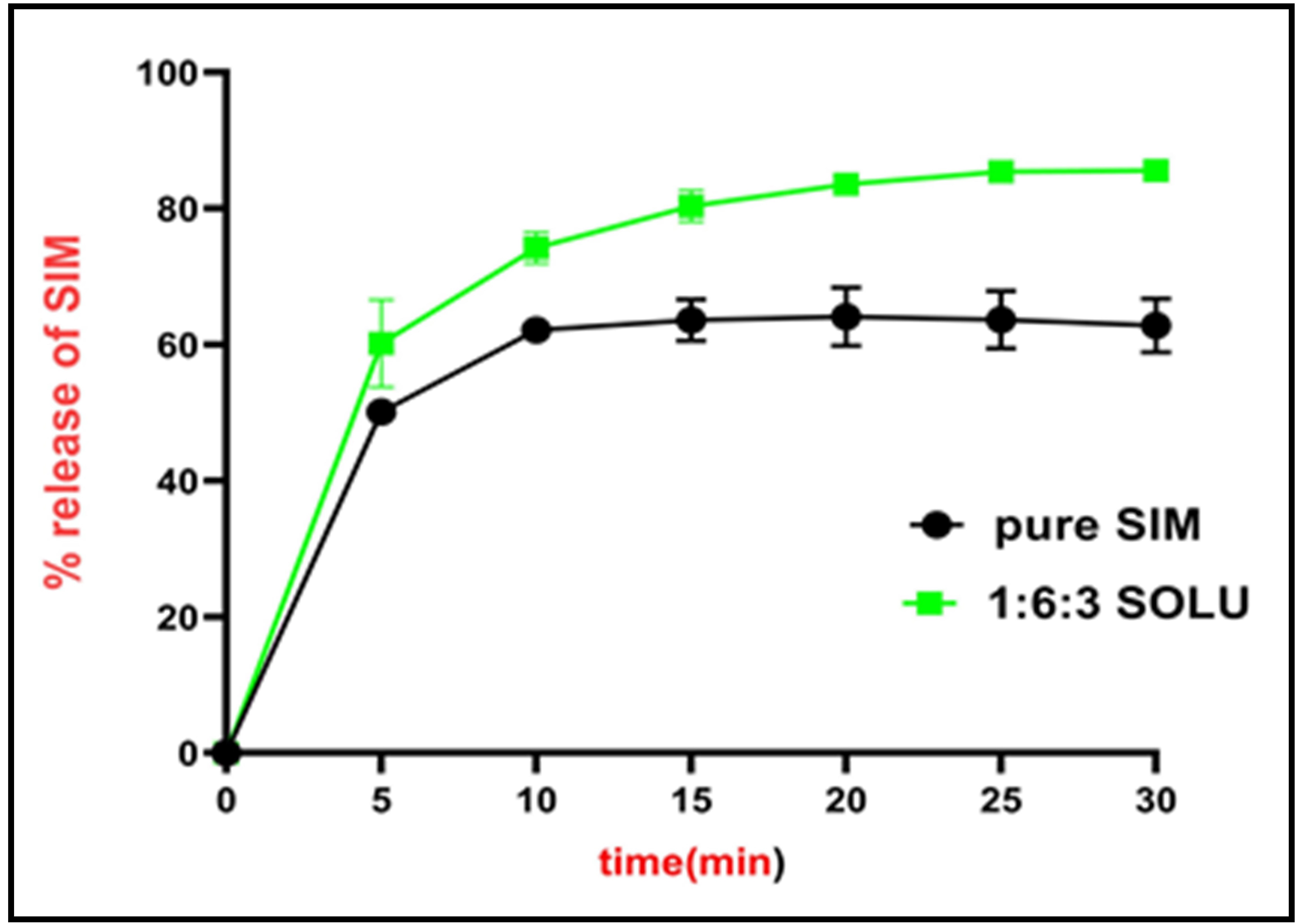
Background: Simvastatin (SIM) is a lipid-lowering agent to prevent disorders caused by clogged blood vessels. Because of its low solubility, it has low bioavailability. The adsorption technique is effective in improving drug solubility and dissolution rate. Objective: To use magnesium aluminum silicate (MAS) as an adsorbent in combination with Soluplus® as a hydrophilic polymer to formulate SIM as immediate-release tablets (IRTs). Methods: We used the solvent evaporation method to make MAS-loaded SIM in the presence of Soluplus®, making sure that the ratio of SIM to MAS to SOLU was 1:6:3. We then used this mixture to make IRTs. Using the direct compression method, we made all of the SIM-IRT formulas. We used diluents like Avicel®PH102, Avicel®PH101, and starch, as well as super disintegrants like Crospovidone (CP), Croscarmellose sodium (CCS), and sodium starch glycolate (SSG). We evaluated these formulas for their weight variation, hardness, friability, disintegration time, drug content, and dissolution profile. Results: We prepared the tablet formula (T5) using MAS-loaded SIM, Avicel®PH102 as a diluent, and CCS 3% as a super disintegrant. This formula showed the shortest disintegration time (0.61 min) and best drug release in phosphate buffer pH 7.0, releasing more than 80% of the drug within 30 minutes. Conclusion: Using suitable excipients, adsorption was an efficient method to enhance the solubility of SIM for preparation as IRTs.
Downloads
References
Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in oral drug delivery. Front Pharmacol. 2021;12:618411. doi: 10.3389/fphar.2021.618411.
Santosh Ubhe T, Gedam P. A brief overview on tablet and it’s types. J Adv Pharmacol. 2020;1(1):21-31.
Sharma N, Pahuja S, Sharma N. Immedıate release tablets: A review. Int J Pharm Sci Res. 2019;10(8):3607. doi: 10.13040/IJPSR.0975-8232.10(8).3607-18.
Hadi MK, Abdulkadir MQ, Sahib HA, Dakhel ZA, Hadi MK, Abdulkadir Q, et al. Hydroxy methylglutaryl-CoA reductase inhibitors (statins), mechanism of action, chemistry, pharmacokinetics and their relative efficacy for improving the lipid profile. J Adv Res Chem Sci. 2021;1(4):52-62.
Finkel R, Clark MA, Cubeddu LX, (Eds.), Lippincott’s Illustrated Reviews: Pharmacology, (4th Ed.), Lippincott Williams & Wilkins; 2009.
Aljuboury R, Shawi NN. Anti-obesity effect of simvastatin and/or omega-3 on obese male wistar rats. Iraqi J Pharm Sci. 2022;31(2):101-112 doi: 10.31351/vol31iss2pp101-112.
Moffat AC, Osselton MD, Widdop B, Watts J, (Eds.), Clarke’s analysis of drugs and poisons, (4th Ed.), London: Pharmaceutical Press; 2011; p.p. 2057–2058.
Shinde S, Kamble SK, Sunita S. Various adsorbent carriers used for enhancing dissolution profile. Trends Drug Del. 2020;7(1):5-9.
Le TT, Elyafi AKE, Mohammed AR, Al-Khattawi A. Delivery of poorly soluble drugs via mesoporous silica: Impact of drug overloading on release and thermal profiles. Pharmaceutics. 2019;11(6):1-16. doi: 10.3390/pharmaceutics11060269.
McCarthy CA, Ahern RJ, Devine KJ, Crean AM. Role of drug adsorption onto the silica surface in drug release from mesoporous silica systems. Mol Pharm. 2018;15(1):141–149. doi: 10.1021/acs.molpharmaceut.7b00778.
Kawano Y, Chen S, Hanawa T. Solubility enhancement of ibuprofen by adsorption onto spherical porous calcium silicate. Pharmaceutics. 2021;13(6):767. doi: 10.3390/pharmaceutics13060767.
Hasson K, Hasson KJ, Ghareeb MM. Evaluatıon of ınnovatıve co-processed addıtıve for dırect compressıon tablets usıng atorvastatın and dıazepam as model drugs. Int J Pharm Pharm Sci. 2016,2(8):201-207.
Aulton ME. Powder flow. Taylor KMG, Aulton ME, (Eds.), Aulton’s pharmaceutics: The design and manufacture of medicine, (6th Ed.), London. Elsevier Ltd, 2022; p 172-183.
United State. Pharmacopeia. The United States Pharmacopeia, USP 43/ The National Formulary 38. In: Rockville, MD: United States Pharmacopeial Convention; 2020.
Mohammed SA, Al-Khedairy EBH. Formulation and in vitro evaluation of taste- masked prednisolone orodispersible tablets. J Fac Med Baghdad. 2023;65(3):192–198. doi: 10.32007/jfacmedbagdad.2057.
Garg MA, Chaturvedi H, Garg A, Rathore US. Post-compressıon evaluatıon parameters for tablets-an overvıew. Eur J Pharm Med Res. 2022;4(11):526-530.
Aziz HA, Entidhar B, Al-Akkam J. Preparation and evaluation of telmisartan solid dispersion as sublingual tablets. J Fac Med Baghdad. 2023;4(65):353-361. doi: 10.32007/jfacmedbagdad.2145.
British Pharmacopoeia Commission. British Pharmacopoeia 2020. London: TSO; 2020.
Borawake PD, Arumugam K, Shınde J V. Formulatıon of solid dispersions for enhancement of solubılıty and dissolution rate of sıimvastatın. Int J Pharm Pharm Sci. 2021;7(13):94-100. doi: 10.22159/ijpps.2021v13i7.41205.
Muselík J, Komersová A, Kubová K, Matzick K, Skalická B. A critical overview of FDA and EMA statistical methods to compare in vitro drug dissolution profiles of pharmaceutical products. Pharmaceutics. 2021;13(10). doi: 10.3390/pharmaceutics13101703.
Choudhari Y, Hoefer H, Libanati C, Monsuur F, McCarthy W. Mesoporous silica drug delivery systems. In: Shcdcha SN, et al., (Eds.), Amorphous Solid Dispersions Advances in Delivery Science and Technology. New York, Springer, 2014; p.p. 665-693. doi: 10.1007/978-1-4939-1598-9_23.
Jagtap RS, Doijad RC, Mohite SK. Adsorption of nifedipine on porous calcium silicate for enhancement of solubility and dissolution rate. Res J Pharm Technol. 2019;12(3):1273. doi: 10.5958/0974-360X.2019.00213.
Maleki A, Hamidi M. Dissolution enhancement of a model poorly water-soluble drug, atorvastatin, with ordered mesoporous silica: Comparison of MSF with SBA-15 as drug carriers. Expert Opin Drug Deliv. 2016;13(2):171-181. doi: 10.1517/17425247.2015.1111335.
Ghyadh BKK, Al-Khedairy EBH. Solubility and dissolution enhancement of atorvastatin calcium using phospholipid solid dispersion technique. Iraqi J Pharm Sci. 2023;32:244-253. doi: 10.31351/vol32issSuppl.pp244-253.
Chaerunisaa AY, Sriwidodo S, Abdassah M. Microcrystalline cellulose as pharmaceutical excipient. In: pharmaceutical formulation design: Recent Practices. 2019. p.p. 1–21. doi: 10.5772/intechopen.88092.
Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients (8th Ed.), London: Pharmaceutical press, 2017; 916–921.
Abduljabbar HH, Abd Alhammid SN. Enhancement of the solubılıty and the dıssolutıon rate of tamoxifen cıtrate soliıd dispersıon using soluplus by solvent evaporatıon technique. Asian J Pharm Clin Res. 2019;12(1):216-221. doi: 10.22159/ajpcr.2018.v12i1.28933.
Hammody ZM, Ghareeb MM. Preparation and characterization of oro-dispersible tablets of bromhexine hydrochloride. J Pharm Sci Res. 2018;10(9):2258-2262.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











